有机化学 ›› 2021, Vol. 41 ›› Issue (4): 1622-1630.DOI: 10.6023/cjoc202010041 上一篇 下一篇
研究论文
刘思展a, 崔明月a, 王博文a, 胡春梅b, 郑莹莹b, 李晶a, 徐学涛b,*( ), 王震a, 王少华a,*(
), 王震a, 王少华a,*( )
)
收稿日期:2020-10-30
修回日期:2020-12-10
发布日期:2020-12-24
通讯作者:
徐学涛, 王少华
基金资助:
Sizhan Liua, Mingyue Cuia, Bowen Wanga, Chunmei Hub, Yingying Zhengb, Jing Lia, Xuetao Xub,*( ), Zhen Wanga, Shaohua Wanga,*(
), Zhen Wanga, Shaohua Wanga,*( )
)
Received:2020-10-30
Revised:2020-12-10
Published:2020-12-24
Contact:
Xuetao Xu, Shaohua Wang
About author:Supported by:文章分享
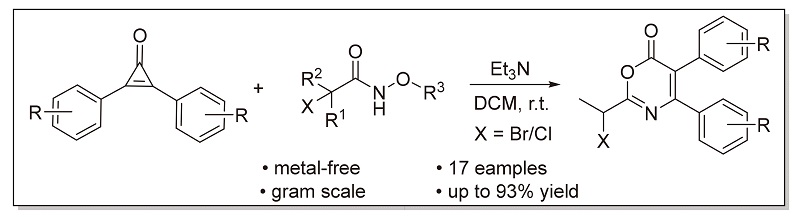
利用环丙烯酮同时具有亲核性、亲电性以及易发生开环反应的特点, 实现了三乙胺促进的环丙烯酮和α-卤代异羟肟酸酯类化合物的[3+3]环加成反应, 快速构筑了6H-1,3-噁嗪-6-酮骨架, 为噁嗪酮类化合物的合成提供了新的思路. 该反应在无金属和温和条件下显示出良好的收率和官能团耐受性, 同时适合克级规模制备.
刘思展, 崔明月, 王博文, 胡春梅, 郑莹莹, 李晶, 徐学涛, 王震, 王少华. 三乙胺促进的环丙烯酮和α-卤代异羟肟酸酯的环化反应合成多取代6H-1,3-噁嗪-6-酮[J]. 有机化学, 2021, 41(4): 1622-1630.
Sizhan Liu, Mingyue Cui, Bowen Wang, Chunmei Hu, Yingying Zheng, Jing Li, Xuetao Xu, Zhen Wang, Shaohua Wang. Triethyl Amine-Promoted Cyclization Reaction between Cyclopropenone and α-Halogenated Hydroxamate for the Synthesis of Polysubstituted 6H-1,3-Oxazin-6-one[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1622-1630.
| Entry | Base | Solvent | Yieldb/% |
|---|---|---|---|
| 1 | DBU | DMF | 33 |
| 2 | DBU | Acetonitrile | 57 |
| 3 | DBU | Ether | 31 |
| 4 | DBU | DMSO | Trace |
| 5 | DBU | n-Heptane | n.d.c |
| 6 | DBU | DCM | 59 |
| 7 | DBU | THF | 58 |
| 8 | DBU | Toluene | 53 |
| 9 | DBU | DCE | Trace |
| 10 | DBU | Dioxane | n.d.c |
| 11 | DMAP | DCM | n.d.c |
| 12 | Et3N | DCM | 59% |
| 13 | TEA | DCM | n.d.c |
| 14 | DIPEA | DCM | 35 |
| 15 | K2CO3 | DCM | 49 |
| 16 | DABCO | DCM | Trace |
| 17 | CS2CO3 | DCM | 32 |
| 18 | NaH | DCM | 45 |
| 19 | Et3Nd | DCM | 44 |
| 20 | Et3Ne | DCM | Trace |
| 21f | Et3Ng | DCM | 70 |
| 22f | Et3Nh | DCM | 75 |
| 23f | Et3Ni | DCM | 67 |
| Entry | Base | Solvent | Yieldb/% |
|---|---|---|---|
| 1 | DBU | DMF | 33 |
| 2 | DBU | Acetonitrile | 57 |
| 3 | DBU | Ether | 31 |
| 4 | DBU | DMSO | Trace |
| 5 | DBU | n-Heptane | n.d.c |
| 6 | DBU | DCM | 59 |
| 7 | DBU | THF | 58 |
| 8 | DBU | Toluene | 53 |
| 9 | DBU | DCE | Trace |
| 10 | DBU | Dioxane | n.d.c |
| 11 | DMAP | DCM | n.d.c |
| 12 | Et3N | DCM | 59% |
| 13 | TEA | DCM | n.d.c |
| 14 | DIPEA | DCM | 35 |
| 15 | K2CO3 | DCM | 49 |
| 16 | DABCO | DCM | Trace |
| 17 | CS2CO3 | DCM | 32 |
| 18 | NaH | DCM | 45 |
| 19 | Et3Nd | DCM | 44 |
| 20 | Et3Ne | DCM | Trace |
| 21f | Et3Ng | DCM | 70 |
| 22f | Et3Nh | DCM | 75 |
| 23f | Et3Ni | DCM | 67 |
| [1] |
(a) Kopelman, P.; Bryson, A.; Hickling, R.; Rissanen, A.; Rossner, S.; Toubro, S.; Valensi, P. Int. J. Obes. 2007, 31,494.
pmid: 18500680 |
|
(b) Yamada, Y.; Kato, T.; Ogino, H.; Ashina, S.; Kato, K. Horm. Metab. Res. 2008, 40,539.
doi: 10.1055/s-2008-1076699 pmid: 18500680 |
|
| [2] |
Stein, R.L.; Strimpler, A.M.; Viscarello, B.R.; Wildonger, R.A.; Mauger, R.C.; Trainor, D.A. Biochemistry 1987, 26,4126.
|
| [3] |
Hays, S.J.; Caprathe, B.W.; Gilmore, J.L.; Amin, N.; Emmerling, M.R.; Michael, W.; Nadimpalli, R.; Nath, R.; Raser, K.J.; Stafford, D.; Watson, D.; Wang, K.; Jaen, J.C. J. Med. Chem. 1998, 41 1060.
doi: 10.1021/jm970394d pmid: 9544206 |
| [4] |
Fenton, G.; Newton, C.G.; Wyman, B.M.; Bagge, P.; Dron, D.I.; Riddell, D.; Jones, G.D. J. Med. Chem. 1989, 32,265.
pmid: 2909740 |
| [5] |
Jarvest, R.L.; Parratt, M.J.; Debouck, C.M.; Gorniak, J.G.; John Jennings, L.; Serafinowska, H.T.; Strickler, J.E. Bioorg. Med. Chem. Lett. 1996, 6,2463.
|
| [6] |
(a) Wu, X.-F.; Schranck, J.; Neumann, H.; Beller, M. Chem.-Eur. J. 2011, 17,12246.
pmid: 28257214 |
|
(b) Liu, Q.; Chen, P.; Liu, G. ACS Catal. 2013, 3,178.
pmid: 28257214 |
|
|
(c) Li, W.; Wu, X.-F. J. Org. Chem. 2014, 79,10410.
doi: 10.1021/jo5020118 pmid: 28257214 |
|
|
(d) Zhang, C.; Li, S.; Bureš, F.; Lee, R.; Ye, X.; Jiang, Z. ACS Catal. 2016, 6,6853.
pmid: 28257214 |
|
|
(e) Song, P.; Yu, P.; Lin, J.-S.; Li, Y.; Yang, N.-Y.; Liu, X.-Y. Org. Lett. 2017, 19,1330.
doi: 10.1021/acs.orglett.7b00178 pmid: 28257214 |
|
|
(f) Niu, B.; Jiang, B.; Yu, L.-Z.; Shi, M. Org. Chem. Front. 2018, 5,1267.
pmid: 28257214 |
|
|
(g) Mohan, R.D.; Jose, A. Asian J. Chem. 2018, 30,1075.
pmid: 28257214 |
|
|
(h) Matsuda, T.; Yamanaka, K.; Tabata, Y.; Shiomi, T. Tetrahedron Lett. 2018, 59,1458.
pmid: 28257214 |
|
| [7] |
Chen, M.; Ren, Z.H.; Wang, Y.Y.; Guan, Z.H. Angew. Chem., Int. Ed. 2013, 52,14196.
|
| [8] |
Karad, S.N.; Chung, W.K.; Liu, R.S. Chem. Sci. 2015, 6,5964.
doi: 10.1039/c5sc01950h pmid: 29861918 |
| [9] |
Jurberg, I.D.; Davies, H.M. L. Org. Lett. 2017, 19,5158.
|
| [10] |
Lang, M.; Wang, J. Org. Chem. Front. 2019, 6,1367.
|
| [11] |
Breslow, R.; Haynie, R.; Mirra, J. J. Am. Chem. Soc. 1959, 81,247.
|
| [12] |
(a) Komatsu, K.; Kitagawa, T. Chem. Rev. 2003, 103,1371.
pmid: 12683786 |
|
(b) Potts, K.T.; Baum, J.S. Chem. Rev. 1974, 74,189.
pmid: 12683786 |
|
|
(c) Prasad, Raiguru, B.; Nayak, S.; Ranjan, Mishra, D.; Das, T.; Mohapatra, S.; Priyadarsini, Mishra, N. Asian J. Org. Chem. 2020, 9,1088.
pmid: 12683786 |
|
| [13] |
(a) Matsumoto, K.; Ikemi, Y.; Hashimoto, S.; Lee, H.S.; Okamoto, Y. J. Org. Chem. 1986, 51,3729.
|
|
(b) Jacobs, C.A.; Dailey, W.P. J. Org. Chem. 1995, 60,7747.
|
|
|
(c) Cordes, M.H. J.; de Gala, S.; Berson, J.A. J. Am. Chem. Soc. 1994, 116,11161.
|
|
| [14] |
(a) Körner, O.; Gleiter, R.; Rominger, F. Synthesis 2009,3259.
pmid: 25764062 |
|
(b) Rivero, A.R.; Fernández, I.; Ramírez de Arellano, C.; Sierra, M.A. J. Org. Chem. 2015, 80,1207.
pmid: 25764062 |
|
|
(c) Wallbaum, J.; Jones, P.G.; Werz, D.B. J. Org. Chem. 2015, 80,3730.
doi: 10.1021/acs.joc.5b00330 pmid: 25764062 |
|
|
(d) Li, L.-H.; Jiang, Y.; Hao, J.; Wei, Y.; Shi, M. Adv. Synth. Catal. 2017, 359,3304.
pmid: 25764062 |
|
| [15] |
(a) Matsuda, T.; Sakurai, Y. Eur. J. Org. Chem. 2013, 2013,4219.
pmid: 30350668 |
|
(b) Yu, S.; Li, X. Org. Lett. 2014, 16,1220.
doi: 10.1021/ol500140e pmid: 30350668 |
|
|
(c) Matsuda, T.; Sakurai, Y. Org. Chem. 2014, 79,2739.
pmid: 30350668 |
|
|
(d) Xie, F.; Yu, S.; Qi, Z.; Li, X. Angew. Chem., Int. Ed. 2016, 55,15351.
pmid: 30350668 |
|
|
(e) Kong, L.; Zhou, X.; Xu, Y.; Li, X. Org. Lett. 2017, 19,3644.
pmid: 30350668 |
|
|
(f) Li, X.; Han, C.; Yao, H.; Lin, A. Org. Lett. 2017, 19,778.
pmid: 30350668 |
|
|
(g) Ren, J.-T.; Wang, J.-X.; Tian, H.; Xu, J.-L.; Hu, H.; Aslam, M.; Sun, M. Org. Lett. 2018, 20,6636.
doi: 10.1021/acs.orglett.8b02612 pmid: 30350668 |
|
|
(h) Shan, L.; Wu, G.; Liu, M.; Gao, W.; Ding, J.; Huang, X.; Wu, H. Org. Chem. Front. 2018, 5,1651.
pmid: 30350668 |
|
|
(i) Liu, Y.; Tian, Y.; Su, K.; Wang, P.; Guo, X.; Chen, B. Org. Chem. Front. 2019, 6,3973.
pmid: 30350668 |
|
| [16] |
(a) Kondo, T.; Taniguchi, R.; Kimura, Y. Synlett 2018, 29,717.
pmid: 31999122 |
|
(b) Haito, A.; Chatani, N. Chem. Commun. 2019, 55,5740.
pmid: 31999122 |
|
|
(c) Bai, D.; Yu, Y.; Guo, H.; Chang, J.; Li, X. Angew. Chem., Int. Ed. 2020, 59,2740.
pmid: 31999122 |
|
|
(d) Nanda, T.; Ravikumar, P.C. Org. Lett. 2020, 22,1368.
pmid: 31999122 |
|
|
(e) Xing, H.; Chen, J.; Shi, Y.; Huang, T.; Hai, L.; Wu, Y. Org. Chem. Front. 2020, 7,672.
pmid: 31999122 |
|
|
(f) Zhou, P.; Yang, W.-T.; Rahman, A.U.; Li, G.; Jiang, B. J. Org. Chem. 2020, 85,360.
pmid: 31999122 |
|
|
(g) Chen, J.; Tang, B.; Liu, X.; Lv, G.; Shi, Y.; Huang, T.; Xing, H.; Guo, X.; Hai, L.; Wu, Y. Org. Chem. Front. 2020, 7,2944.
pmid: 31999122 |
|
| [17] |
(a) Vanos, C.; Lambert, T. Angew. Chem., Int. Ed. 2011, 50,12222.
pmid: 32643378 |
|
(b) Wei, Y.; Zhao, W.-T.; Yang, Y.-L.; Zhang, Z.; Shi, M. ChemCatChem 2015, 7,3340.
pmid: 32643378 |
|
|
(c) Shih, H.-W.; Prescher, J.A. J. Am. Chem. Soc. 2015, 137,10036.
pmid: 32643378 |
|
|
(d) Aly, A.A.; Ramadan, M.; Al-Aziz, M.A.; Fathy, H.M.; Bräse, S.; Brown, A.B.; Nieger, M. J. Chem. Res. 2016, 40,637.
pmid: 32643378 |
|
|
(e) Cunha, S.; Serafim, J.C.; de Santana, L.L. B.; Damasceno, F.; Correia, J.T. M.; Santos, A.O.; Oliveira, M.; Ribeiro, J.; Amparo, J.; Costa, S.L. J. Heterocycl. Chem. 2017, 54,3700.
pmid: 32643378 |
|
|
(f) El-Sheref, E.M. J. Sulfur Chem. 2017, 38,625.
pmid: 32643378 |
|
|
(g) Li, X.; Han, C.; Yao, H.; Lin, A. Org. Lett. 2017, 19,778.
pmid: 32643378 |
|
|
(h) Xu, J.; Cao, J.; Fang, C.; Lu, T.; Du, D. Org. Chem. Front. 2017, 4,560.
pmid: 32643378 |
|
|
(i) Reitel, K.; Kriis, K.; Järving, I.; Kanger, T. Chem. Heterocycl. Compd. 2018, 54,929.
pmid: 32643378 |
|
|
(j) Matsuda, T.; Tabata, Y.; Suzuki, H. New J. Chem. 2018, 42,19178.
pmid: 32643378 |
|
|
(k) Wu, J.; Gao, W.-X.; Huang, X.-B.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Org. Lett. 2020, 22,5555.
doi: 10.1021/acs.orglett.0c01914 pmid: 32643378 |
|
|
(l) Liu, S.-W.; Yuan, C.; Jiang, X.-F.; Wang, X.-X.; Cui, H.-L. Asian J. Org. Chem. 2020, 9,82.
pmid: 32643378 |
|
|
(m) Yang, Y.-F.; Huang, X.-B.; Gao, W.-X.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Org. Biomol. Chem. 2020, 18,5822.
pmid: 32643378 |
|
|
(n) Jamshaid, F.; Kondakal, V.V. R.; Newman, C.D.; Dobson, R.; João, H.; Rice, C.R.; Mwansa, J.M.; Thapa, B.; Hemming, K. Tetrahedron 2020,131570.
pmid: 32643378 |
|
| [18] |
(a) Zhu, B.Z.; Shao, B.; Li, F.; Liu, Y.X.; Huang, C.H. Acta Chim. Sinica 2015, 73,765. (in Chinese)
|
|
( 朱本占, 邵波, 李锋, 刘玉祥, 黄春华, 化学学报, 2015, 73,765.)
|
|
|
(b) Gu, Y.Y.; Lü, X.Q.; Ma, X.D.; Zhang, H.J.; Ji, Y.Y.; Ding, W.J.; Shen, L. Chin. J. Org. Chem. 2020, 40,95. (in Chinese)
|
|
|
( 顾依钰, 吕晓庆, 马晓东, 张浩健, 嵇媛媛, 丁婉婧, 沈立, 有机化学, 2020, 40,95.)
|
|
| [19] |
Peart, P.A.; Tovar, J.D. J. Org. Chem. 2010, 75,5689.
pmid: 20704438 |
| [20] |
Jeffrey, C.S.; Barnes, K.L.; Eickhoff, J.A.; Carson, C.R. J. Am. Chem. Soc. 2011, 133,7688.
doi: 10.1021/ja201901d pmid: 21534633 |
| [21] |
(a) Foucaud, A.; Bakouetila, M. Synthesis 1987,854.
pmid: 27199108 |
|
(b) Li, C.; Jiang, K.; Ouyang, Q.; Liu, T.-Y.; Chen, Y.-C. Org. Lett. 2016, 18,2738.
pmid: 27199108 |
| [1] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [2] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [3] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [4] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [5] | 李靖鹏, 黄顺桃, 杨棋, 李伟强, 刘腾, 黄超. 利用连续流动技术合成(Z)-N-乙烯基取代N,O-缩醛[J]. 有机化学, 2023, 43(4): 1550-1558. |
| [6] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| [7] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [8] | 南宁, 吴双, 秦景灏, 李金恒. 基于硅烷化启动的环化反应研究进展[J]. 有机化学, 2023, 43(10): 3414-3453. |
| [9] | 桑田, 贾帆, 何静, 李春天, 刘岩, 刘平. I2催化β-酮腈与1H-吡唑-5-胺的环化反应[J]. 有机化学, 2023, 43(1): 195-201. |
| [10] | 刘东汉, 鲁席杭, 柴张梦洁, 杨浩琦, 孙瑜琳, 余富朝. 构建2H-吡咯-2-酮骨架的研究进展[J]. 有机化学, 2023, 43(1): 57-73. |
| [11] | 王川川, 马志伟, 侯学会, 杨龙华, 陈亚静. N-Ts氰胺在有机合成中的研究与应用[J]. 有机化学, 2023, 43(1): 74-93. |
| [12] | 覃小婷, 邹宁, 农彩梅, 莫冬亮. 九元氮杂环化合物合成最新研究进展[J]. 有机化学, 2023, 43(1): 130-155. |
| [13] | 刘浩阳, 孙爽爽, 马献力, 陈艳艳, 徐燕丽. 可见光促进异腈插入反应合成硒代螺环[吲哚-3,3'-喹啉]衍生物[J]. 有机化学, 2022, 42(9): 2867-2876. |
| [14] | 王苛莉, 黄静, 刘伟, 伍智林, 于贤勇, 蒋俊, 何卫民. 由N-(2-丙炔基)苯胺和磺酰氯直接合成3-砜基喹啉[J]. 有机化学, 2022, 42(8): 2527-2534. |
| [15] | 张智鑫, 翟彤仪, 朱伯汉, 钱鹏程, 叶龙武. 无金属催化炔酰胺分子内[4+2]环化反应合成四氢吲哚衍生物[J]. 有机化学, 2022, 42(5): 1501-1508. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||