Acta Chimica Sinica ›› 2019, Vol. 77 ›› Issue (10): 1017-1023.DOI: 10.6023/A19060203 Previous Articles Next Articles
Article
投稿日期:2019-06-08
发布日期:2019-09-05
通讯作者:
张永丽
E-mail:zxm581212@163.com
基金资助:Received:2019-06-08
Published:2019-09-05
Contact:
Zhang, Yongli
E-mail:zxm581212@163.com
Supported by:Share
Yang, Bo, Zhang, Yongli. Synergistic Removal of Co-contamination by Heterogeneous Fenton System: Chemical Conversion, pH Effect and Mechanism Analysis[J]. Acta Chimica Sinica, 2019, 77(10): 1017-1023.


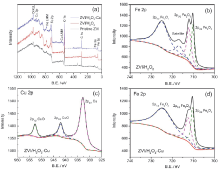

| Element (photo electro core level) | Peak position/eV | Assignment | Area | FWHM/eV | |
|---|---|---|---|---|---|
| ZVI/H2O2 | ZVI/H2O2-Cu | ||||
| Fe 2p | 724.1 | 2p1/2 Fe2O3 | 5473.1 | 7.323 | |
| 716.7 | Satellite of Fe2O3 | 1373.7 | 4.47 | ||
| 712.1 | 2p3/2 Fe2O3 | 2281.4 | 2.333 | ||
| 710.2 | 2p3/2 Fe3O4 | 1825.9 | 1.539 | ||
| 724.1 | 2p1/2 Fe2O3 | 5264.5 | 9.995 | ||
| 712.1 | 2p3/2 Fe2O3 | 1735.5 | 3013 | ||
| 710.2 | 2p3/2 Fe3O4 | 1584.5 | 1.707 | ||
| Cu 2p | — | 952.7 | 2p1/2 CuO | 128.8 | 1.745 |
| — | 942.0 | 2p3/2 CuO | 160.1 | 2.025 | |
| — | 932.7 | 2p3/2 Cu0 | 794.2 | 2.808 | |
| Element (photo electro core level) | Peak position/eV | Assignment | Area | FWHM/eV | |
|---|---|---|---|---|---|
| ZVI/H2O2 | ZVI/H2O2-Cu | ||||
| Fe 2p | 724.1 | 2p1/2 Fe2O3 | 5473.1 | 7.323 | |
| 716.7 | Satellite of Fe2O3 | 1373.7 | 4.47 | ||
| 712.1 | 2p3/2 Fe2O3 | 2281.4 | 2.333 | ||
| 710.2 | 2p3/2 Fe3O4 | 1825.9 | 1.539 | ||
| 724.1 | 2p1/2 Fe2O3 | 5264.5 | 9.995 | ||
| 712.1 | 2p3/2 Fe2O3 | 1735.5 | 3013 | ||
| 710.2 | 2p3/2 Fe3O4 | 1584.5 | 1.707 | ||
| Cu 2p | — | 952.7 | 2p1/2 CuO | 128.8 | 1.745 |
| — | 942.0 | 2p3/2 CuO | 160.1 | 2.025 | |
| — | 932.7 | 2p3/2 Cu0 | 794.2 | 2.808 | |
| [1] |
Fu, F.; Dionysiou, D. D.; Liu, H. J. Hazard. Mater. 2014, 267, 194.
doi: 10.1016/j.jhazmat.2013.12.062 |
| [2] |
Yamaguchi, R.; Kurosu, S.; Suzuki, M.; Kawase, Y . Chem. Eng. J. 2018, 334, 1537.
doi: 10.1016/j.cej.2017.10.154 |
| [3] |
Wei, X. Y.; Gao, N. Y.; Li, C. J.; Deng, Y.; Zhou, S. Q.; Li, L. Chem. Eng. J. 2016, 285, 660.
doi: 10.1016/j.cej.2015.08.120 |
| [4] |
Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L . Chemosphere 2017, 174, 665.
doi: 10.1016/j.chemosphere.2017.02.019 |
| [5] |
Ling, R.; Chen, J. P.; Shao, J.; Reinhard, M. Water Res. 2018, 134, 44.
doi: 10.1016/j.watres.2018.01.065 |
| [6] |
Liu, J.; Ou, C.; Han, W.; Faheem, F.; Shen, J.; Bi, H.; Sun, X.; Li, J.; Wang, L. RSC Adv. 2015, 5, 57444.
doi: 10.1039/C5RA08487C |
| [7] |
Cho, Y.; Choi, S. I . Chemosphere 2010, 81, 940.
doi: 10.1016/j.chemosphere.2010.07.054 |
| [8] |
Zheng, Z. Q.; Lu, G. N.; Wang, R.; Huang, K. B.; Tao, X. Q.; Yang, Y. L.; Zou, M. Y.; Xie, Y. Y.; Yin, H. Environ. Pollut. 2019, 245, 780.
doi: 10.1016/j.envpol.2018.11.064 |
| [9] |
Gu, T. H.; Shi, J. M.; Hua, Y. L.; Liu, J.; Wang, W.; Zhang, W. X . Acta Chim. Sinica 2017, 75, 991.
doi: 10.6023/A17070345 |
|
( 顾天航, 石君明, 滑熠龙, 刘静, 王伟, 张伟贤, 化学学报, 2017, 75, 991.)
doi: 10.6023/A17070345 |
|
| [10] |
Li, Z.; Luo, S. Q.; Yang, Y.; Chen, J. W . Chemosphere 2019, 216, 499.
doi: 10.1016/j.chemosphere.2018.10.133 |
| [11] |
Cai, C.; Wang, L. G.; Gao, H.; Hou, L. W.; Zhang, H. J. Environ. Sci. 2014, 26, 1267.
doi: 10.1016/S1001-0742(13)60598-7 |
| [12] |
Cao, C. J.; Liu, X. G.; Ju, X. R.; Chen, X. R . Acta Phys.-Chim. Sin. 2013, 29, 2475.
doi: 10.3866/PKU.WHXB201310101 |
|
( 曹崇江, 刘晓庚, 鞠兴荣, 陈晓荣 , 物理化学学报, 2013, 29, 2475.)
doi: 10.3866/PKU.WHXB201310101 |
|
| [13] |
Huang, X. Y.; Wang, W.; Lin, L.; Zhang, W. X . Acta Chim. Sinica2017, 75, 529.
doi: 10.6023/A17020051 |
|
( 黄潇月, 王伟, 凌岚, 张伟贤, 化学学报, 2017, 75, 529.)
doi: 10.6023/A17020051 |
|
| [14] |
Liu, J.; Gu, T. H.; Wang, W.; Liu, A. R.; Zhang, W. X . Acta Chim. Sinica 2019, 77, 121.
doi: 10.6023/A18100412 |
|
( 刘静, 顾天航, 王伟, 刘爱荣, 张伟贤, 化学学报, 2019, 77, 121.)
doi: 10.6023/A18100412 |
|
| [15] | Li, J. H.; Lin, C. F.; Qin, W.; Xiao, X. B.; Wei, L. Acta Phys.-Chim. Sin. 2016, 32, 2717. |
| ( 李继红, 林常枫, 覃吴, 肖显斌, 魏利 , 物理化学学报, 2016, 32, 2717.) | |
| [16] |
Jiang, X.; Qiao, J.; Lo, I. M.; Wang, L.; Guan, X.; Lu, Z.; Zhou, G.; Xu, C. J. Hazard. Mater. 2015, 283, 880.
doi: 10.1016/j.jhazmat.2014.10.044 |
| [17] |
Fu, R. B.; Yang, Y. P.; Xu, Z.; Zhang, X.; Guo, X. P.; Bi, D. S . Chemosphere 2015, 138, 726.
doi: 10.1016/j.chemosphere.2015.07.051 |
| [18] |
Diao, Z.-H.; Xu, X.-R.; Jiang, D.; Liu, J.-J.; Kong, L.-J.; Li, G.; Zuo, L.-Z.; Wu, Q.-H . Chem. Eng. J. 2017, 315, 167.
doi: 10.1016/j.cej.2017.01.006 |
| [19] |
Liu, C. M.; Diao, Z. H.; Huo, W. Y.; Kong, L. J.; Du, J. J. Environ. Pollut. 2018, 239, 698.
doi: 10.1016/j.envpol.2018.04.084 |
| [20] |
Sleiman, N.; Deluchat, V.; Wazne, M.; Mallet, M.; Courtin-Nomade, A.; Kazpard, V.; Baudu, M. Colloids Surf., A 2017, 514, 1.
doi: 10.1016/j.colsurfa.2016.11.014 |
| [21] |
Li, H.; Wan, J.; Ma, Y.; Wang, Y.; Huang, M. Chem. Eng. J. 2014, 237, 487.
doi: 10.1016/j.cej.2013.10.035 |
| [22] |
Xu, C. H.; Zhang, B. L.; Wang, Y. H.; Shao, Q. Q.; Zhou, W. Z.; Fan, D. M.; Bandstra, J. Z.; Shi, Z. Q.; Tratnyek, P. G. Environ. Sci. Technol. 2016, 50, 11879.
doi: 10.1021/acs.est.6b03184 |
| [23] |
Zhang, L.; Shao, Q.; Xu, C . J. Clean. Prod. 2019, 213, 753.
doi: 10.1016/j.jclepro.2018.12.183 |
| [24] |
Liu, W.; Sun, W.; Borthwick, A. G. L.; Wang, T.; Li, F.; Guan, Y . J. Hazard. Mater. 2016, 317, 385.
doi: 10.1016/j.jhazmat.2016.06.002 |
| [25] |
Grosvenor, A. P.; Kobe, B. A.; Biesinger, M. C.; McIntyre, N. S. Surf. Interface Anal. 2004, 36, 1564.
doi: 10.1002/(ISSN)1096-9918 |
| [26] |
Choi, K.; Lee, W. J. Hazard. Mater. 2012, 211-212, 146.
doi: 10.1016/j.jhazmat.2011.10.056 |
| [27] |
Luo, F.; Chen, Z.; Megharaj, M.; Naidu, R. Chem. Eng. J. 2016, 294, 290.
doi: 10.1016/j.cej.2016.03.005 |
| [28] |
Gao, Y. Y.; Li, H. X.; Ou, Z. Z.; Hao, P.; Li, Y.; Yang, G. Q. Acta Phys.-Chim. Sin. 2011, 27, 2469.
doi: 10.3866/PKU.WHXB20110939 |
|
( 高云燕, 李海霞, 欧植泽, 郝平, 李嫕, 杨国强 , 物理化学学报, 2011, 27, 2469.)
doi: 10.3866/PKU.WHXB20110939 |
|
| [29] | Gotic, M.; Music, S. J. Mol. Struct. 2007, 834, 445. |
| [30] |
Dong, H.; He, Q.; Zeng, G.; Tang, L.; Zhang, L.; Xie, Y.; Zeng, Y.; Zhao, F. Chem. Eng. J. 2017, 316, 410.
doi: 10.1016/j.cej.2017.01.118 |
| [31] |
Cai, X.; Gao, Y.; Sun, Q.; Chen, Z.; Megharaj, M.; Naidu, R. Chem. Eng. J. 2014, 244, 19.
doi: 10.1016/j.cej.2014.01.040 |
| [32] |
Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I. M.; He, D.; Dong, Water Res. 2015, 75, 224.
doi: 10.1016/j.watres.2015.02.034 |
| [33] |
Wang, N.; Zheng, T.; Jiang, J.; Wang, P . Chem. Eng. J. 2015, 260, 386.
doi: 10.1016/j.cej.2014.09.002 |
| [34] |
Zhou, P.; Zhang, J.; Zhang, Y.; Zhang, G.; Li, W.; Wei, C.; Liang, J.; Liu, Y.; Shu, S. J. Hazard. Mater. 2018, 344, 1209.
doi: 10.1016/j.jhazmat.2017.11.023 |
| [1] | Huang Danwei, He Jia, Gu Yawei, He Feng. Mechanochemically Sulfidated Zero Valent Iron as an Efficient Fenton-like Catalyst for Degradation of Organic Contaminants [J]. Acta Chim. Sinica, 2017, 75(9): 866-872. |
| [2] | . Theoretical Study on the Gas-Phase Mechanism of the Reaction of N2O with CO Circularly Catalyzed by Ir+ [J]. Acta Chimica Sinica, 2008, 66(24): 2669-2674. |
| [3] | MU Guan-Nan, LI Xiang-Hong, QU Qing, ZHOU Jun. Synergistic Effect on Corrosion Inhibition by Cerium(IV) Ion and Sodium Molybdate for Cold Rolled Steel in Hydrochloric Acid Solution [J]. Acta Chimica Sinica, 2004, 62(24): 2386-2390. |
| [4] | CUI Jian, LI Zhao-Long, HONG Xiao-Yin. Studies on the Antioxidant of Anthraquinone Derivatives and β-Carotene [J]. Acta Chimica Sinica, 2004, 62(12): 1095-1100. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
