Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (5): 590-597.DOI: 10.6023/A22010014 Previous Articles Next Articles
Special Issue: 中国科学院青年创新促进会合辑
Article
张瑾a,c, 丁湘浓a, 刘红超a, 樊栋a, 徐舒涛a,*( ), 魏迎旭a, 刘中民a,b,c
), 魏迎旭a, 刘中民a,b,c
投稿日期:2022-01-09
发布日期:2022-05-31
通讯作者:
徐舒涛
作者简介:基金资助:
Jin Zhanga,c, Xiangnong Dinga, Hongchao Liua, Dong Fana, Shutao Xua( ), Yingxu Weia, Zhongmin Liua,b,c
), Yingxu Weia, Zhongmin Liua,b,c
Received:2022-01-09
Published:2022-05-31
Contact:
Shutao Xu
About author:Supported by:Share
Jin Zhang, Xiangnong Ding, Hongchao Liu, Dong Fan, Shutao Xu, Yingxu Wei, Zhongmin Liu. Study on the Framework Aluminum Distributions of HMOR Zeolite and Identification of Active Sites for Dimethyl Ether Carbonylation Reaction※[J]. Acta Chimica Sinica, 2022, 80(5): 590-597.
| Sample | CXRD/% | Si/Al ratio | Surface area/(m2•g-1) | Pore volume/(cm3•g-1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| BET | Micro* | Ext* | Total | Micro* | Meso* | ||||
| NH4MOR | 100 | 7.3 | — | — | — | — | — | — | |
| HMOR-450-4 | 90.3 | 9.1 | 446.3 | 409.7 | 36.5 | 0.23 | 0.19 | 0.06 | |
| HMOR-500-4 | 91.1 | 9.7 | 432.9 | 395.9 | 37.0 | 0.22 | 0.18 | 0.06 | |
| HMOR-550-4 | 90.8 | 10.9 | 454.5 | 414.1 | 40.3 | 0.24 | 0.20 | 0.06 | |
| HMOR-600-4 | 90.9 | 12.5 | 400.2 | 366.0 | 34.2 | 0.21 | 0.18 | 0.05 | |
| Sample | CXRD/% | Si/Al ratio | Surface area/(m2•g-1) | Pore volume/(cm3•g-1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| BET | Micro* | Ext* | Total | Micro* | Meso* | ||||
| NH4MOR | 100 | 7.3 | — | — | — | — | — | — | |
| HMOR-450-4 | 90.3 | 9.1 | 446.3 | 409.7 | 36.5 | 0.23 | 0.19 | 0.06 | |
| HMOR-500-4 | 91.1 | 9.7 | 432.9 | 395.9 | 37.0 | 0.22 | 0.18 | 0.06 | |
| HMOR-550-4 | 90.8 | 10.9 | 454.5 | 414.1 | 40.3 | 0.24 | 0.20 | 0.06 | |
| HMOR-600-4 | 90.9 | 12.5 | 400.2 | 366.0 | 34.2 | 0.21 | 0.18 | 0.05 | |
| Sample | 1H MAS NMR/(mmol•g–1) | FTIRa/(mmol•g–1) | |
|---|---|---|---|
| Total | 8MR | 12MR | |
| HMOR-450-4 | 1.62 | 0.98 | 0.64 |
| HMOR-500-4 | 1.86 | 1.00 | 0.86 |
| HMOR-550-4 | 1.36 | 0.83 | 0.53 |
| HMOR-600-4 | 0.9 | 0.52 | 0.38 |
| Sample | 1H MAS NMR/(mmol•g–1) | FTIRa/(mmol•g–1) | |
|---|---|---|---|
| Total | 8MR | 12MR | |
| HMOR-450-4 | 1.62 | 0.98 | 0.64 |
| HMOR-500-4 | 1.86 | 1.00 | 0.86 |
| HMOR-550-4 | 1.36 | 0.83 | 0.53 |
| HMOR-600-4 | 0.9 | 0.52 | 0.38 |
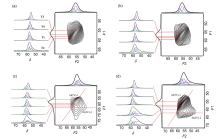

| Sample | Al(Ⅳ)-1 | Al(Ⅳ)-2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T2 | T1 | T4 | T3 | |||||||||||||
| (%) | mmol•g–1 | (%) | mmol•g–1 | (%) | mmol•g–1 | (%) | mmol•g–1 | (%) | mmol•g–1 | |||||||
| HMOR-450-4 | 9.93 | 0.1639 | 18.11 | 0.299 | 40.69 | 0.671 | 31.27 | 0.516 | — | — | ||||||
| HMOR-500-4 | 10.20 | 0.1589 | 18.37 | 0.286 | 40.63 | 0.633 | 30.8 | 0.480 | — | — | ||||||
| HMOR-550-4 | 9.95 | 0.1394 | 15.38 | 0.215 | 24.80 | 0.347 | 23.66 | 0.331 | 26.2 | 0.367 | ||||||
| HMOR-600-4 | 9.11 | 0.1125 | 14.80 | 0.183 | 24.16 | 0.298 | 13.73 | 0.170 | 38.20 | 0.471 | ||||||
| Sample | Al(Ⅳ)-1 | Al(Ⅳ)-2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T2 | T1 | T4 | T3 | |||||||||||||
| (%) | mmol•g–1 | (%) | mmol•g–1 | (%) | mmol•g–1 | (%) | mmol•g–1 | (%) | mmol•g–1 | |||||||
| HMOR-450-4 | 9.93 | 0.1639 | 18.11 | 0.299 | 40.69 | 0.671 | 31.27 | 0.516 | — | — | ||||||
| HMOR-500-4 | 10.20 | 0.1589 | 18.37 | 0.286 | 40.63 | 0.633 | 30.8 | 0.480 | — | — | ||||||
| HMOR-550-4 | 9.95 | 0.1394 | 15.38 | 0.215 | 24.80 | 0.347 | 23.66 | 0.331 | 26.2 | 0.367 | ||||||
| HMOR-600-4 | 9.11 | 0.1125 | 14.80 | 0.183 | 24.16 | 0.298 | 13.73 | 0.170 | 38.20 | 0.471 | ||||||
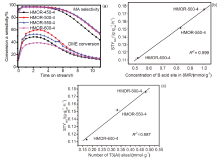
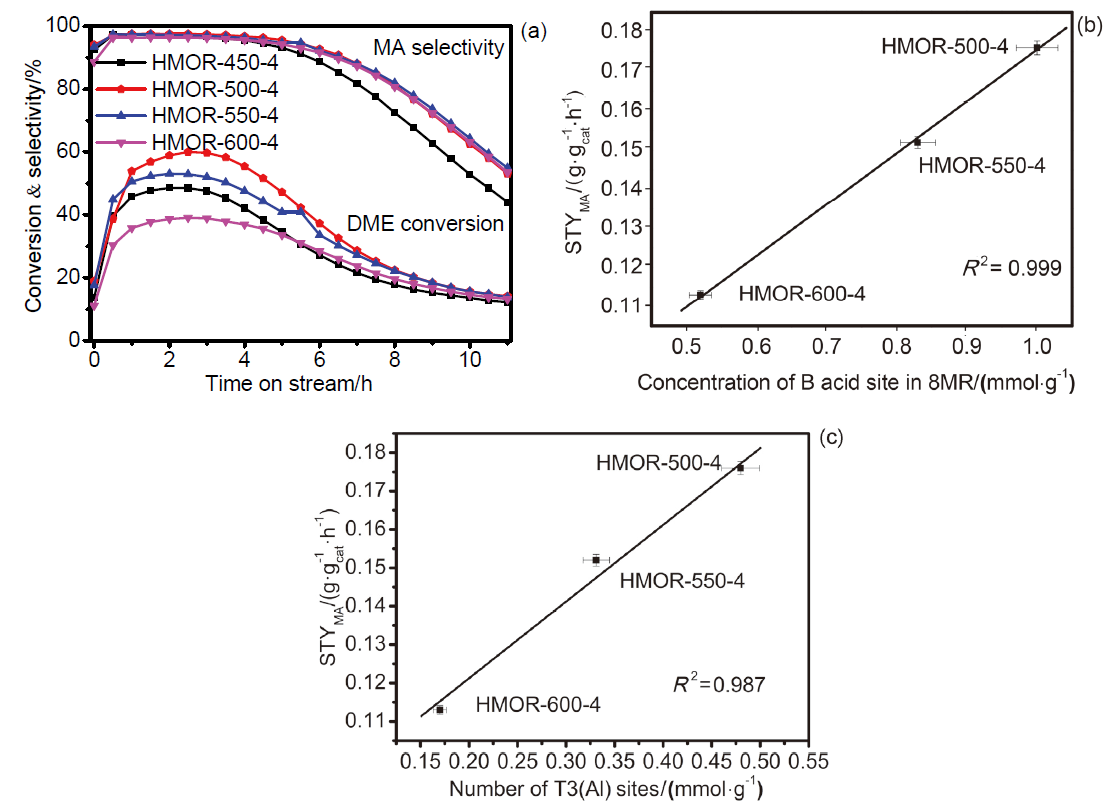
| [1] |
Sun, D.; Sun, B.; Pei, Y.; Yan, S. R.; Fan, K. N.; Qiao, M. H.; Zhang, X. X.; Zong, B. N. Acta Chim. Sinica 2021, 79, 771. (in Chinese)
doi: 10.6023/A20120563 |
|
(孙冬, 孙博, 裴燕, 闫世润, 范康年, 乔明华, 张晓昕, 宗保宁, 化学学报, 2021, 79, 771.)
doi: 10.6023/A20120563 |
|
| [2] |
Zhang, M. T.; Yan, T. T.; Dai, W. L.; Guan, N. J.; Li, L. D. Acta Chim. Sinica 2020, 78, 1404. (in Chinese)
doi: 10.6023/A20080346 |
|
(张梦婷, 颜婷婷, 戴卫理, 关乃佳, 李兰冬, 化学学报, 2020, 78, 1404.)
doi: 10.6023/A20080346 |
|
| [3] |
Yao, X. T.; Huang, X.; Lin, Y. X.; Liu, Y. M. Acta Chim. Sinica 2020, 78, 1111. (in Chinese)
doi: 10.6023/A20060246 |
|
(姚旭婷, 黄鑫, 林玉霞, 刘月明, 化学学报, 2020, 78, 1111.)
doi: 10.6023/A20060246 |
|
| [4] |
Liu, Y. H.; Zhao, N.; Xian, H.; Cheng, Q. P.; Tan, Y. S.; Tsubaki, N.; Li, X. G. ACS Appl. Mater. Inter. 2015, 7, 8398.
doi: 10.1021/acsami.5b01905 |
| [5] |
Li, Y.; Sun, Q.; Huang, S. Y.; Cheng, Z. Z.; Cai, K.; Lv, J.; Ma, X. B. Catal. Today 2018, 311, 81.
doi: 10.1016/j.cattod.2017.08.050 |
| [6] |
Zhao, N.; Cheng, Q. P.; Lyu, S. S.; Guo, L. H.; Tian, Y.; Ding, T.; Xu, J.; Ma, X. B.; Li, X. G. Catal. Today. 2020, 339, 86.
doi: 10.1016/j.cattod.2019.01.013 |
| [7] |
Huo, H.; Peng, L. M.; Gan, Z. H.; Grey, C. P. J. Am. Chem. Soc. 2012, 134, 9708.
doi: 10.1021/ja301963e |
| [8] |
Lukyanov, D. B.; Vazhnova, T.; Cherkasov, N.; Casci, J. L.; Birtill, J. J. J. Phys. Chem. C 2014, 118, 23918.
doi: 10.1021/jp5086334 |
| [9] |
Cai, K.; Huang, S. Y.; Li, Y.; Cheng, Z. Z.; Lv, J.; Ma, X. B. ACS Sustain. Chem. Eng. 2019, 7, 2027.
doi: 10.1021/acssuschemeng.8b04388 |
| [10] |
Yi, X. F.; Xiao, Y.; Li, G. C.; Liu, Z. Q.; Chen, W.; Liu, S. B.; Zheng, A. M. Chem. Mater. 2020, 32, 1332.
doi: 10.1021/acs.chemmater.0c00005 |
| [11] |
Gong, K.; Liu, Z. M.; Liang, L. X.; Zhao, Z. C.; Guo, M. L.; Liu, X. B.; Han, X. W.; Bao, X. H.; Hou, G. J. J. Phys. Chem. Lett. 2021, 12, 2413.
doi: 10.1021/acs.jpclett.0c03610 pmid: 33661009 |
| [12] |
Cao, K. P.; Fan, D.; Gao, M. B.; Fan, B. H.; Chen, N.; Wang, L. Y.; Tian, P.; Liu, Z. M. ACS Catal. 2021, 12, 1.
doi: 10.1021/acscatal.1c04966 |
| [13] |
Bajpai, P. K. Zeolites 1986, 6, 2.
doi: 10.1016/0144-2449(86)90002-3 |
| [14] |
Bajpai, P. K.; Rao, M. S.; Gokhale, K. V. G. K. Ind. Eng. Chem. Prod. Res. Dev. 1981, 20, 721.
doi: 10.1021/i300004a026 |
| [15] |
Bhan, A.; Allian, A. D.; Sunley, G. J.; Law, D. J.; Iglesia, E. J. Am. Chem. Soc. 2007, 129, 4919.
doi: 10.1021/ja070094d |
| [16] |
Boronat, M.; Martinez-Sanchez, C.; Law, D.; Corma, A. J. Am. Chem. Soc. 2008, 130, 16316.
doi: 10.1021/ja805607m |
| [17] |
Cheung, P.; Bhan, A.; Sunley, G. J.; Iglesia, E. Angew. Chem., Int. Ed. 2006, 45, 1617.
doi: 10.1002/anie.200503898 |
| [18] |
Cheung, P.; Bhan, A.; Sunley, G.; Law, D.; Iglesia, E. J. Catal. 2007, 245, 110.
doi: 10.1016/j.jcat.2006.09.020 |
| [19] |
Jiang, Y. J.; Hunger, M.; Wang, W. J. Am. Chem. Soc. 2006, 128, 11679.
doi: 10.1021/ja061018y |
| [20] |
Chu, Y. Y.; Lo, A. Y.; Wang, C.; Deng, F. J. Phys. Chem. C 2019, 123, 15503.
doi: 10.1021/acs.jpcc.9b01874 |
| [21] |
Blasco, T.; Boronat, M.; Concepcion, P.; Corma, A.; Law, D.; Vidal-Moya, J. A. Angew. Chem., Int. Ed. 2007, 46, 3938.
|
| [22] |
He, T.; Ren, P. J.; Liu, X. C.; Xu, S. T.; Han, X. W.; Bao, X. H. Chem. Commun. 2015, 51, 16868.
doi: 10.1039/C5CC07201H |
| [23] |
Li, B. J.; Xu, J.; Han, B.; Wang, X. M.; Qi, G. D.; Zhang, Z. F.; Wang, C.; Deng, F. J. Phys. Chem. C 2013, 117, 5840.
doi: 10.1021/jp400331m |
| [24] |
Xue, H. F.; Huang, X. M.; Ditzel, E.; Zhan, E. S.; Ma, M.; Shen, W. J. Ind. Eng. Chem. Res. 2013, 52, 11510.
doi: 10.1021/ie400909u |
| [25] |
Wang, S. R.; Guo, W. W.; Zhu, L. J.; Wang, H. X.; Qiu, K. Z.; Cen, K. F. J. Phys. Chem. C 2014, 119, 524.
doi: 10.1021/jp511543x |
| [26] |
Liu, S. P.; Liu, H. C.; Ma, X. G.; Liu, Y.; Zhu, W. L.; Liu, Z. M. Catal. Sci. Technol. 2020, 10, 4663.
doi: 10.1039/D0CY00125B |
| [27] |
Cao, K. P.; Fan, D.; Li, L. Y; Fan, B. H.; Wang, L. Y.; Zhu, D. L.; Wang, Q. Y.; Tian, P.; Liu, Z. M. ACS Catal. 2020, 10, 3372.
doi: 10.1021/acscatal.9b04890 |
| [28] |
Wang, X. S.; Li, R. J.; Yu, C. C.; Liu, Y. X.; Zhang, L. Y.; Xu, C. M.; Zhou, H. J. Fuel 2019, 239, 794.
doi: 10.1016/j.fuel.2018.10.147 |
| [29] |
Reule, A. A. C.; Sawada, J. A.; Sema gina, N. J. Catal. 2017, 349, 98.
doi: 10.1016/j.jcat.2017.03.010 |
| [30] |
Li, Y.; Li, Z. H.; Huang, S. Y.; Cai, K.; Qu, Z.; Zhang, J. F.; Wang, Y.; Ma, X. B. ACS Appl. Mater. Inter. 2019, 11, 24000.
doi: 10.1021/acsami.9b03588 |
| [31] |
Paul, G.; Bisio, C.; Braschi, I.; Cossi, M.; Gatti, G.; Gianotti, E.; Marchese, L. Chem. Soc. Rev. 2018, 47, 5684.
doi: 10.1039/c7cs00358g pmid: 30014075 |
| [32] |
Chen, X.; Fu, Y. Y.; Yue, B.; He, H. Y. Chin. J. Magn. Reson. 2021, 38, 491. (in Chinese)
|
|
(陈欣, 付颖懿, 岳斌, 贺鹤勇, 波谱学杂志, 2021, 38, 491.)
|
|
| [33] |
Gao, S. S.; Xu, S. T.; Wei, Y. X.; Liu, Z. M. Chin. J. Magn. Reson. 2021, 38, 433. (in Chinese)
|
|
(高树树, 徐舒涛, 魏迎旭, 刘中民, 波谱学杂志, 2021, 38, 433.)
|
|
| [34] |
Xao, Y.; Xia, C. J.; Yi, X. F.; Liu, F. Q.; Liu, S. B.; Zheng, A. M. Chin. J. Magn. Reson. 2021, 38, 571. (in Chinese)
|
|
肖瑶, 夏长久, 易先锋, 刘凤庆, 刘尚斌, 郑安民, 波谱学杂志, 2021, 38, 571.)
|
|
| [35] |
Chen, H. D.; Kong, H. Y.; Zhao, Z. C.; Zhang, W. P. Chin. J. Magn. Reson. 2021, 38, 543. (in Chinese)
|
|
(陈翰迪, 孔海宇, 赵侦超, 张维萍, 波谱学杂志, 2021, 38, 543.)
|
|
| [36] |
Yang, W. J.; Huang, J. Chin. J. Magn. Reson. 2021, 38, 460. (in Chinese)
|
|
(杨文杰, 黄骏, 波谱学杂志, 2021, 38, 460.)
|
|
| [37] |
Wang, Y. X. Wang, Q.; Xu, J.; Xia, Q. H.; Deng, F. Chin. J. Magn. Reson. 2021, 38, 514. (in Chinese)
|
|
(王永祥, 王强, 徐君, 夏清华, 邓风, 波谱学杂志, 2021, 38, 514.)
|
|
| [38] |
Xia, X. F.; Zhang, W. J.; Lin, Z. Y.; Ke, X. K.; Wen, Y. J.; Wang, F.; Chen, J. C.; Peng, L. M. Chin. J. Magn. Reson. 2021, 38, 533. (in Chinese)
|
|
(夏锡锋, 张文静, 林芝晔, 柯晓康, 温玉洁, 王芳, 陈俊超, 彭路明, 波谱学杂志, 2021, 38, 533.)
|
|
| [39] |
Fyfe, C. A.; Gobbi, G. C.; Murphy, W. J.; Ozubko, R. S.; Slack, D. A. J. Am. Chem. Soc. 1984, 106, 4435.
doi: 10.1021/ja00328a024 |
| [40] |
Yokoi, T.; Mochizuki, H.; Namba, S.; Kondo, J. N.; Tatsumi, T. J. Phys. Chem. C 2015, 119, 15303.
doi: 10.1021/acs.jpcc.5b03289 |
| [41] |
Kneller, J. M.; Pietraß, T.; Ott, K. C.; Labouriau, A. Micropor. Mesopor. Mater. 2003, 62, 121.
doi: 10.1016/S1387-1811(03)00400-1 |
| [42] |
Ravi, M.; Sushkevich, V. L.; van Bokhoven, J. A. Chem. Sci. 2021, 12, 4094.
doi: 10.1039/D0SC06130A |
| [43] |
Medek, A.; Harwood, J. S.; Frydman, L. J. Am. Chem. Soc. 1995, 117, 12779.
doi: 10.1021/ja00156a015 |
| [44] |
Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z. H.; Hoatson, G. Magn. Reason. Chem. 2002, 40, 70.
|
| [45] |
Hu, J. Z.; Wan, C.; Vjunov, A.; Wang, M.; Zhao, Z. C.; Hu, M. Y.; Camaioni, D. M.; Lercher, J. A. J. Phys. Chem. C. 2017, 121, 12849.
doi: 10.1021/acs.jpcc.7b03517 |
| [46] |
Frydman, L.; Harwood, J. S. J. Am. Chem. Soc. 1995, 117, 5367.
doi: 10.1021/ja00124a023 |
| [47] |
Sarv, P.; Tuherm, T.; Lippmaa, E.; Keskinen, K.; Root, A. J. Phys. Chem. 1995, 99, 13763.
doi: 10.1021/j100038a003 |
| [48] |
Maache, M.; Janin, A.; Lavalley, J. C.; Benazzi, E. Zeolites 1995, 15, 507.
doi: 10.1016/0144-2449(95)00019-3 |
| [49] |
Pastvova, J.; Pilar, R.; Moravkova, J.; Kaucky, D.; Rathousky, J.; Sklenak, S.; Sazama, P. Appl. Catal. A. 2018, 562, 159.
doi: 10.1016/j.apcata.2018.05.035 |
| [50] |
Wakabayashi, F.; Kondo, J.; Wada, A.; Domen, K.; Hirose, C. J. Phys. Chem. 1996, 100, 4154.
doi: 10.1021/jp9522775 |
| [51] |
Zholobenko, V. L.; Makarova, M. A.; Dwyer, J. J. Phys. Chem. 2002, 97, 5962.
doi: 10.1021/j100124a030 |
| [52] |
Engelhardt, G.; Kentgens, A. P. M.; Koller, H.; Samoson, A. Solid State Nucl. Magn. Reson. 1999, 15, 171.
pmid: 10672941 |
| [53] |
Liu, R. S.; Fan, B. H.; Zhang, W. N.; Wang, Y. L.; Qi, L.; Xu, S. T.; Yu, Z. X.; Wei, Y. X.; Liu, Z. M. Angew. Chem., Int. Ed. 2022, e202116990.
|
| [54] |
Jeffroy, M.; Nieto-Draghi, C.; Boutin, A. Chem. Mater. 2017, 29, 513.
doi: 10.1021/acs.chemmater.6b03011 |
| [55] |
Chen, K.; Horstmeier, S.; Nguyen, V. T.; Wang, B.; Crossley, S. P.; Pham, T.; Gan, Z.; Hung, I.; White, J. L. J. Am. Chem. Soc. 2020, 142, 7514.
doi: 10.1021/jacs.0c00590 |
| [56] |
Chen, K.; Gan, Z.; Horstmeier, S.; White, J. L. J. Am. Chem. Soc. 2021, 143, 6669.
doi: 10.1021/jacs.1c02361 |
| [57] |
Bodart, P.; Nagy, J. B.; Debras, G.; Gabelica, Z.; Jacobs, P. A. J. Phys. Chem. 1986, 90, 5183.
doi: 10.1021/j100412a058 |
| [58] |
Debras, G.; Nagy, J. B.; Gabelica, Z.; Bodart, P.; Jacobs, P. A. Chem. Lett. 1983, 12, 199.
doi: 10.1246/cl.1983.199 |
| [59] |
Schroeder, K. P.; Sauer, J. J. Phys. Chem. 1993, 97, 6579.
|
| [60] |
Amoureux, J. P.; Fernandez, C.; Steuernagel, S. J. Magn. Reson. A 1996, 123, 116.
doi: 10.1006/jmra.1996.0221 |
| [1] | Chen Chunhui, Zhan Ensheng, Li Yong, Shen Wenjie. Enantioselective Hydrogenation of α,β-Unsaturated Carboxylic Acids:Effects of Palladium Particle Size and Support Acidic Property [J]. Acta Chimica Sinica, 2013, 71(11): 1505-1510. |
| [2] | MO Chun-Li, DICKO Cedric, SHAO Zheng-Zhong, CHEN Xin. Time-Resolved Total Reflectance FT-IR Spectroscopy Characterization on Conformational Change of Silk Fibroin Induced by Acetic Acid [J]. Acta Chimica Sinica, 2009, 67(22): 2641-2644. |
| [3] | . Hydrothermal Stability of Natural CXN Zeolite II. Influence of Steaming Temperature on the Properties of NH4-Type Zeolite [J]. Acta Chimica Sinica, 2009, 67(18): 2067-2073. |
| [4] | ZHAO Hai-Hong XIE Guo-Yong*2 LIU Zhen-Yu2 LIU You-Zhi. A Combined in-situ Diffuse Reflectance FTIR and On-line Mass Spectroscopy Study of Surface Acidity and Reactivity over a CuO/Al2O3 Catalyst [J]. Acta Chimica Sinica, 2008, 66(9): 1021-1027. |
| [5] | . Binder-free ZSM-5 Zeolite Catalysts Modified with Framework De-alumination [J]. Acta Chimica Sinica, 2008, 66(19): 2099-2106. |
| [6] |
HUANG, Bao-Hua* LI, Zi-Jin WANG, Yan-Fei ZHANG, Kun FANG, Yan-Xiong* . Esterification Catalyzed by Brönsted Acidic Ionic Liquids [J]. Acta Chimica Sinica, 2008, 66(15): 1837-1844. |
| [7] |
YAO Rui-Ping,ZHANG Ming-Jin*,1,2,YANG Jun2,YI De-Lian XU Jun,DENG Feng2,YUE Yong2,YE Chao-Hui2. Preparation of SO3/γ-Al2O3 Solid Acid Catalyst and Characterization of Its Structure and Acidity [J]. Acta Chimica Sinica, 2005, 63(4): 269-273. |
| [8] | ZHOU Wei-Zheng, LIN De-Chang, ZHONG Ying, GUO Juan, WANG Ti, LONG Ying-Cai. Studies of THF-FER Zeolite Ⅳ. Acidity and Catalytic Property for Skeletal Isomerization of C5 Olefins [J]. Acta Chimica Sinica, 2004, 62(8): 833-838. |
| [9] | HE Jie, FAN Yi-Ning, QIU Jin-Heng, CHEN Yi. Dispersion State and Catalytic Properties of Niobia Species on the Surface of Nb2O5/TiO2 Catalysts [J]. Acta Chimica Sinica, 2004, 62(14): 1311-1317. |
| [10] | Chen Jianshe;Lu Gui;Wei Danyi;Yao Kemin;Shen Lianfang. Studies on the acidity properties of Eu(Ⅲ) complexes with α-amino acids by ^1^3C NMR [J]. Acta Chimica Sinica, 1998, 56(9): 892-899. |
| [11] | XIA SHIJUN;CHENG DEPING. Studies on the directional reaction of silver coordination compounds with hydrazine [J]. Acta Chimica Sinica, 1990, 48(3): 251-255. |
| [12] | YANG SHUXUN;TONG HUAXIANG;WANG WENXIANG. Kinetic study on the reaction system of Tl^3^+-H2O2-Fe^2^+ [J]. Acta Chimica Sinica, 1989, 47(9): 831-837. |
| [13] | LIU CHENGMIN;WANG YAHUI;CHI XIZENG;LIANG SHUQUAN. The additivity of the absorbances of Europium (or other Lanthanides)-Bivalent metal ions-xylenol orange [J]. Acta Chimica Sinica, 1989, 47(6): 563-567. |
| [14] | PANG WENQIN;QIU SHILUN;DI BIN;ZHOU FENGQI. Synthesis and characterization of Ga-ZSM-5 zeolite in a slightly acidic medium [J]. Acta Chimica Sinica, 1989, 47(5): 476-480. |
| [15] | LU QIN;WANG GUOXIONG;ZANG YAN;ZENG CHENG. Correlation between acid strengths and quantum chemical parameters of some oxygen-containing acids [J]. Acta Chimica Sinica, 1989, 47(3): 284-287. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||