Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (8): 1057-1060.DOI: 10.6023/A22030130 Previous Articles Next Articles
Communication
投稿日期:2022-03-24
发布日期:2022-09-01
通讯作者:
薛敏
基金资助:
Minggang Wua, Yong Yangb, Min Xuea( )
)
Received:2022-03-24
Published:2022-09-01
Contact:
Min Xue
Supported by:Share
Minggang Wu, Yong Yang, Min Xue. Tetraaminopillar[5]arene Dimers: Synthesis, Structure and Properties[J]. Acta Chimica Sinica, 2022, 80(8): 1057-1060.
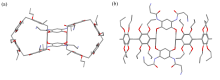



| [1] |
(a) Diederich, F.; Stang, P. J.; Tykwinski, R. R. Modern Supramolecular Chemistry: Strategies for Macrocycle Synthesis, Wiley-VCH, Berlin, 2008, p. 1.
|
|
(b) Steed, J. W.; Atwood, J. L. Supramolecular Chemistry, John Wiley & Sons, Ltd., New York, 2009, p. 1.
|
|
|
(c) Li, J.; Han, Y.; Chen, C. Chin. J. Org. Chem. 2020, 40, 3714. (in Chinese)
doi: 10.6023/cjoc202005007 |
|
|
(李晶, 韩莹, 陈传峰, 有机化学, 2020, 40, 3714.)
doi: 10.6023/cjoc202005007 |
|
| [2] |
(a) Xue, M.; Yang, Y.; Chi, X.; Yan, X.; Huang, F. Chem. Rev. 2015, 115, 7398.
doi: 10.1021/cr5005869 |
|
(b) Xia, D.; Wang, P.; Ji, X.; Khashab, N. M.; Sessler, J. L.; Huang, F. Chem. Rev. 2020, 120, 6070.
doi: 10.1021/acs.chemrev.9b00839 |
|
|
(c) Han, X. N.; Han, Y.; Chen, C. F. J. Am. Chem. Soc. 2020, 142, 8262.
doi: 10.1021/jacs.0c00624 |
|
| [3] |
(a) Jiang, Y.; Guo, J. B.; Chen, C. F. Chem. Commun. 2010, 46, 5536.
doi: 10.1039/c0cc00999g |
|
(b) Evans, N. H.; Serpell, C. J.; Beer, P. D. Angew. Chem., Int. Ed. 2011, 50, 2507.
doi: 10.1002/anie.201007741 |
|
|
(c) Li, C.; Han, K.; Li, J.; Zhang, H.; Ma, J.; Shu, X.; Chen, Z.; Weng, L.; Jia, X. Org. Lett. 2012, 14, 42.
doi: 10.1021/ol2027834 |
|
|
(d) Hu, W. B.; Xie, C. D.; Hu, W. J.; Zhao, X. L.; Liu, Y. A.; Huo, J. C.; Li, J. S.; Jiang, B.; Wen, K. J. Org. Chem. 2015, 80, 7994.
doi: 10.1021/acs.joc.5b01038 |
|
|
(e) Zhou, Y.; Jie, K.; Shi, B.; Yao, Y. Chem. Commun. 2015, 51, 11112.
doi: 10.1039/C5CC02886H |
|
|
(f) Sun, Y.; Wang, J.; Yao, Y. Chem. Commun. 2017, 53, 165.
doi: 10.1039/C6CC08452D |
|
|
(g) Ding, Y.; Alimi, L. O.; Moosa, B.; Maaliki, C.; Jacquemin, J.; Huang, F.; Khashab, N. M. Chem. Sci. 2021, 12, 5315.
doi: 10.1039/D1SC00440A |
|
| [4] |
(a) Zhang, Z.; Luo, Y.; Chen, J.; Dong, S.; Yu, Y.; Ma, Z.; Huang, F. Angew. Chem., Int. Ed. 2011, 50, 1397.
doi: 10.1002/anie.201006693 |
|
(b) Song, N.; Chen, D. X.; Qiu, Y. C.; Yang, X. Y.; Xu, B.; Tian, W.; Yang, Y. W. Chem. Commun. 2014, 50, 8231.
doi: 10.1039/c4cc03105a |
|
|
(c) Meng, L. B.; Li, D.; Xiong, S.; Hu, X. Y.; Wang, L.; Li, G. Chem. Commun. 2015, 51, 4643.
doi: 10.1039/C5CC00398A |
|
|
(d) Huo, B.; Li, B.; Su, H.; Zeng, X.; Xu, K.; Cui, L. Chin. J. Org. Chem. 2019, 39, 1990. (in Chinese)
doi: 10.6023/cjoc201811003 |
|
|
(霍博超, 李斌, 苏杭, 曾宪强, 徐凯迪, 崔雷, 有机化学, 2019, 39, 1990.)
doi: 10.6023/cjoc201811003 |
|
|
(e) Xiao, T.; Zhou, L.; Sun, X.-Q.; Huang, F.; Lin, C.; Wang, L. Chin. Chem. Lett. 2020, 31, 1.
doi: 10.1016/j.cclet.2019.05.011 |
|
| [5] |
(a) Wu, J.; Sun, S.; Feng, X.; Shi, J.; Hu, X. Y.; Wang, L. Chem. Commun. 2014, 50, 9122.
doi: 10.1039/C4CC03127J |
|
(b) Yue, S. Y.; Zhou, Y. J.; Yao, Y.; Xue, M. Acta Chim. Sinica 2014, 72, 1053. (in Chinese)
doi: 10.6023/A14080579 |
|
|
(岳诗雨, 周玉娟, 姚勇, 薛敏, 化学学报, 2014, 72, 1053.)
doi: 10.6023/A14080579 |
|
|
(c) Wang, Q.; Cheng, M.; Zhao, Y.; Wu, L.; Jiang, J.; Wang, L.; Pan, Y. Chem. Commun. 2015, 51, 3623.
doi: 10.1039/C5CC00130G |
|
|
(d) Song, N.; Chen, D. X.; Xia, M. C.; Qiu, X. L.; Ma, K.; Xu, B.; Tian, W.; Yang, Y. W. Chem. Commun. 2015, 51, 5526.
doi: 10.1039/C4CC08205B |
|
|
(e) Chen, J.; Zhang, Y.; Meng, Z.; Guo, L.; Yuan, X.; Zhang, Y.; Chai, Y.; Sessler, J. L.; Meng, Q.; Li, C. Chem. Sci. 2020, 11, 6275.
doi: 10.1039/D0SC01756F |
|
| [6] |
(a) Wu, X.; Duan, Q. P.; Ni, M. F.; Hu, X. Y.; Wang, L. Y. Chin. J. Org. Chem. 2014, 34, 437. (in Chinese)
|
|
(吴旋, 段群鹏, 倪梦飞, 胡晓玉, 王乐勇, 有机化学, 2014, 34, 437.)
doi: 10.6023/cjoc201312018 |
|
|
(b) Hu, W. B.; Hu, W. J.; Zhao, X. L.; Liu, Y. A.; Li, J. S.; Jiang, B.; Wen, K. Chem. Commun. 2015, 51, 13882.
doi: 10.1039/C5CC05623C |
|
|
(c) Li, S. H.; Zhang, H. Y.; Xu, X.; Liu, Y. Nat. Commun. 2015, 6, 7590.
doi: 10.1038/ncomms8590 |
|
|
(d) Zhang, R.; Wang, C.; Sun, J.; Yan, C.; Yao, Y. Chin. J. Org. Chem. 2019, 39, 3483. (in Chinese)
doi: 10.6023/cjoc201906006 |
|
|
(张润淼, 王陈威, 孙晶, 颜朝国, 姚勇, 有机化学, 2019, 39, 3483.)
doi: 10.6023/cjoc201906006 |
|
|
(e) Liang, H.; Hua, B.; Xu, F.; Gan, L. S.; Shao, L.; Huang, F. J. Am. Chem. Soc. 2020, 142, 19772.
doi: 10.1021/jacs.0c10570 |
|
|
(f) Dong, J. H.; Li, J. J.; Wang, H.; Liu, B. X.; Peng, B.; Chen, J. Z.; Lin, S. L. Acta Chim. Sinica 2021, 79, 803. (in Chinese)
doi: 10.6023/A21030105 |
|
|
(董锦辉, 李进杰, 王赫, 刘彬秀, 彭博, 陈健壮, 林绍梁, 化学学报, 2021, 79, 803.)
doi: 10.6023/A21030105 |
|
| [7] |
(a) Li, Z. Y.; Zhang, Y.; Zhang, C. W.; Chen, L. J.; Wang, C.; Tan, H.; Yu, Y.; Li, X.; Yang, H. B. J. Am. Chem. Soc. 2014, 136, 8577.
doi: 10.1021/ja413047r |
|
(b) Chen, J.; Liu, X.; Han, B.; Ding, J.; Zhang, Y.; Lin, Q.; Yao, H.; Wei, T. Chin. J. Org. Chem. 2018, 38, 2741. (in Chinese)
doi: 10.6023/cjoc201805003 |
|
|
(陈进发, 刘茜, 韩冰冰, 丁金东, 张有明, 林奇, 姚虹, 魏太保, 有机化学, 2018, 38, 2741.)
doi: 10.6023/cjoc201805003 |
|
|
(c) Li, B.; Wang, B.; Huang, X.; Dai, L.; Cui, L.; Li, J.; Jia, X.; Li, C. Angew. Chem., Int. Ed. 2019, 58, 3885.
doi: 10.1002/anie.201813972 |
|
|
(d) Liu, J.; Sun, X. W.; Huang, T. T.; Zhang, Y. M.; Yao, H.; Wei, T. B.; Lin, Q. Chin. J. Chem. 2021, 39, 3421.
doi: 10.1002/cjoc.202100583 |
|
| [8] |
(a) Si, W.; Chen, L.; Hu, X. B.; Tang, G.; Chen, Z.; Hou, J. L.; Li, Z. T. Angew. Chem., Int. Ed. 2011, 50, 12564.
doi: 10.1002/anie.201106857 pmid: 23362942 |
|
(b) Chen, L.; Si, W.; Zhang, L.; Tang, G.; Li, Z. T.; Hou, J. L. J. Am. Chem. Soc. 2013, 135, 2152.
doi: 10.1021/ja312704e pmid: 23362942 |
|
|
(c) Yan, Z. J.; Wang, D.; Ye, Z.; Fan, T.; Wu, G.; Deng, L.; Yang, L.; Li, B.; Liu, J.; Ma, T.; Dong, C.; Li, Z. T.; Xiao, L.; Wang, Y.; Wang, W.; Hou, J. L. J. Am. Chem. Soc. 2020, 142, 15638.
doi: 10.1021/jacs.0c00601 pmid: 23362942 |
|
|
(d) Yan, Z. J.; Li, Y. W.; Yang, M.; Fu, Y. H.; Wen, R.; Wang, W.; Li, Z. T.; Zhang, Y.; Hou, J. L. J. Am. Chem. Soc. 2021, 143, 11332.
doi: 10.1021/jacs.1c06000 pmid: 23362942 |
|
|
(e) Li, Y. W.; Fu, Y. H.; Hou, J. L. Chin. J. Chem. 2022, 40, 1293.
doi: 10.1002/cjoc.202100836 pmid: 23362942 |
|
| [9] |
Wan, K.; Gao, S. C.; Fang, X.; Xu, M. Y.; Yang, Y.; Xue, M. Chem. Commun. 2020, 56, 10155.
doi: 10.1039/D0CC04375C |
| [10] |
Shu, X.; Chen, S.; Li, J.; Chen, Z.; Weng, L.; Jia, X.; Li, C. Chem. Commun. 2012, 48, 2967.
doi: 10.1039/c2cc00153e |
| [11] |
(a) Thordarson, P. Chem. Soc. Rev. 2010, 40, 1305.
doi: 10.1039/C0CS00062K |
|
(b) Hibbert, D. B.; Thordarson, P. Chem. Commun. 2016, 52, 12792.
doi: 10.1039/C6CC03888C |
|
| [12] |
Capici, C.; Gattuso, G.; Notti, A.; Parisi, M. F.; Pappalardo, S.; Brancatelli, G.; Geremia, S. J. Org. Chem. 2012, 77, 9668.
doi: 10.1021/jo301730m |
| [1] | Ying Wei, Ping Zhou, Xin Chen, Qiujing Bao, Linghai Xie. Research Progress on Organic Nanohoops/Nanogrids [J]. Acta Chimica Sinica, 2023, 81(3): 289-308. |
| [2] | Xiuqing Huang, Qi Zhang. A Gourd-shaped Organometallic Coordination Cage: Synthesis and Selective Binding of Two Drug Molecules [J]. Acta Chimica Sinica, 2023, 81(3): 217-221. |
| [3] | Xiaomao Tian, Yuequn Lin, Han Zhu, Chao Huang, Bixue Zhu. Enantiomers Identification of Penicillamine by Chiral Mono-Schiff Base Macrocycles [J]. Acta Chimica Sinica, 2023, 81(1): 20-28. |
| [4] | Chuanzhi Liu, Fen Li, Jingjing Wang, Xiaolu Zhao, Tingmei Zhang, Xin Huang, Mengli Wu, Zhiyuan Hu, Xinming Liu, Zhanting Li. Self-assembly of Supramolecular Planar Macrocycle Driven by Intermolecular Halogen Bonding [J]. Acta Chimica Sinica, 2022, 80(10): 1365-1368. |
| [5] | Tengfei Yan, Shengda Liu, Yichen Luo, Yingping Zou, Junqiu Liu. Research Progress on the Macrocycle-Derived Artificial Transmembrane Ion Channels [J]. Acta Chimica Sinica, 2021, 79(8): 999-1007. |
| [6] | Bo Yifan, Liu Yuyu, Chang Yongzheng, Li Yinxiang, Zhang Xiaofei, Song Chunyuan, Xu Weifeng, Cao Hongtao, Huang Wei. Theoretical and Experimental Studies on Raman Spectroscopy of Cyclic Fluorene-Based Strained Semiconductors [J]. Acta Chim. Sinica, 2019, 77(5): 442-446. |
| [7] | Zhou Li, Xu Lijin, Gong Hanyuan. Pattern and Protonation Effect of Benzene Dicarboxylate Anion Species in Self-assembly System [J]. Acta Chimica Sinica, 2014, 72(4): 447-455. |
| [8] | Han Chengyou, Zhang Zibin, Chi Xiaodong, Zhang Mingming, Yu Guocan, Huang Feihe. Synthesis of 1,4-Bis(n-propoxy)pillar[7]arene and Its Host-guest Chemistry [J]. Acta Chimica Sinica, 2012, 70(17): 1775-1778. |
| [9] | Yang Dengke, Zeng Zhijian, Chen Mujuan, Pan Shaowu, Yang Yu, Li Mei, Lei Chunyan, Jiang Lasheng. Studies on the Complexation of di-Amide Based Macrocycles with Pyridine N-oxides [J]. Acta Chimica Sinica, 2012, 70(12): 1385-1393. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||