Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (3): 295-302.DOI: 10.6023/A23110508 Previous Articles Next Articles
Article
韩晶, 廖润华*( ), 邓文强, 梁博宇, 周雨晴, 任帅, 洪燕*(
), 邓文强, 梁博宇, 周雨晴, 任帅, 洪燕*( )
)
投稿日期:2023-11-21
发布日期:2024-02-19
基金资助:
Jing Han, Runhua Liao*( ), Wenqiang Deng, Boyu Liang, Yuqing Zhou, Shuai Ren, Yan Hong*(
), Wenqiang Deng, Boyu Liang, Yuqing Zhou, Shuai Ren, Yan Hong*( )
)
Received:2023-11-21
Published:2024-02-19
Contact:
*E-mail: Supported by:Share
Jing Han, Runhua Liao, Wenqiang Deng, Boyu Liang, Yuqing Zhou, Shuai Ren, Yan Hong. Study on Performance of Copper Doped Carbon Nitride Electrocatalyzing Nitrate to Produce Ammonia[J]. Acta Chimica Sinica, 2024, 82(3): 295-302.
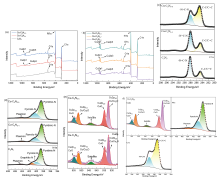



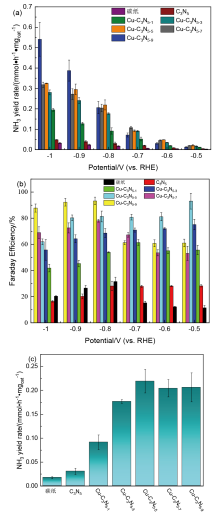

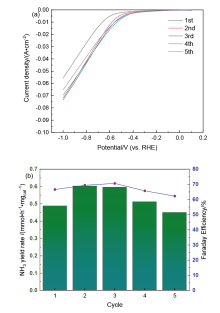
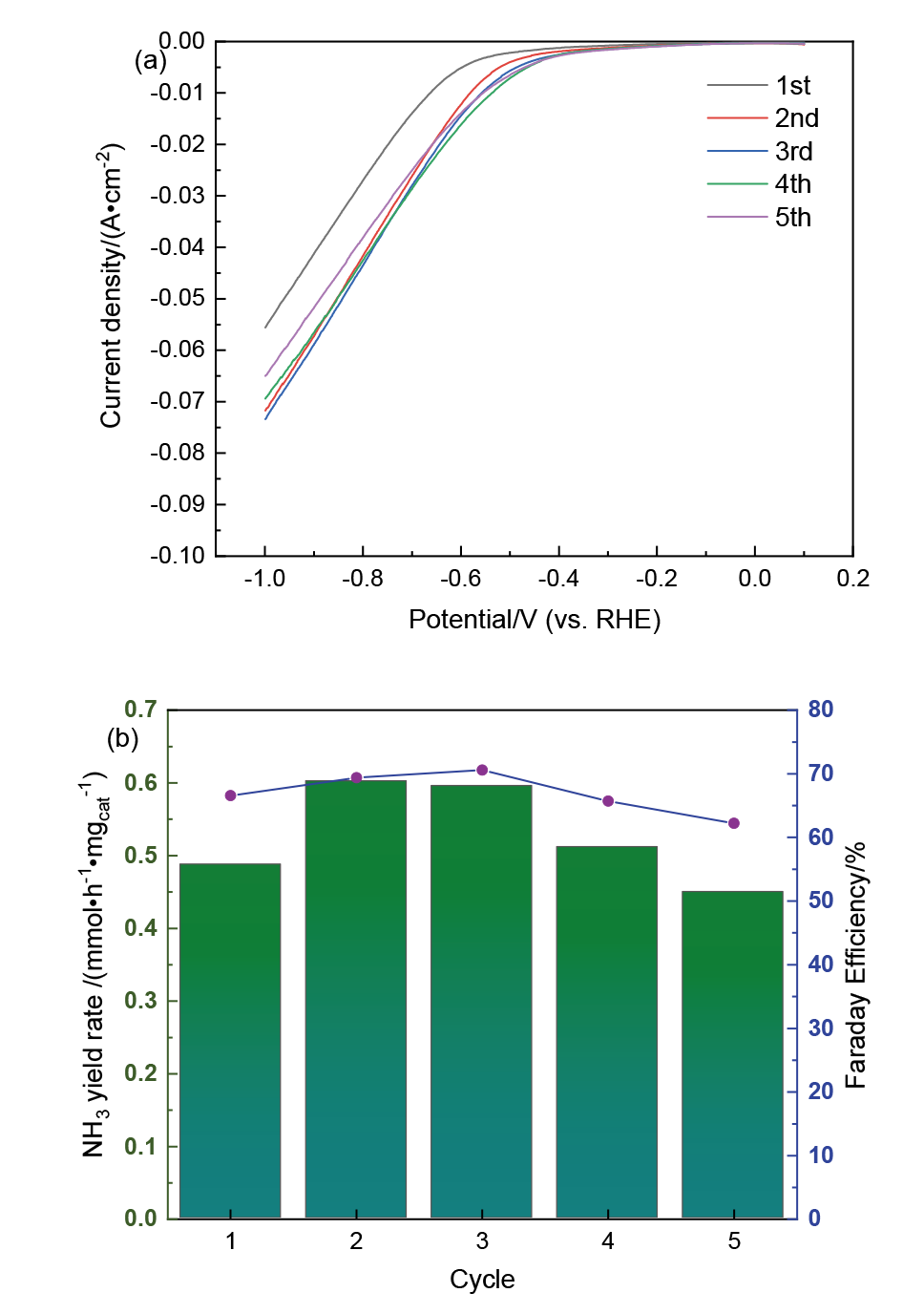
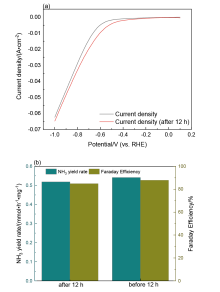
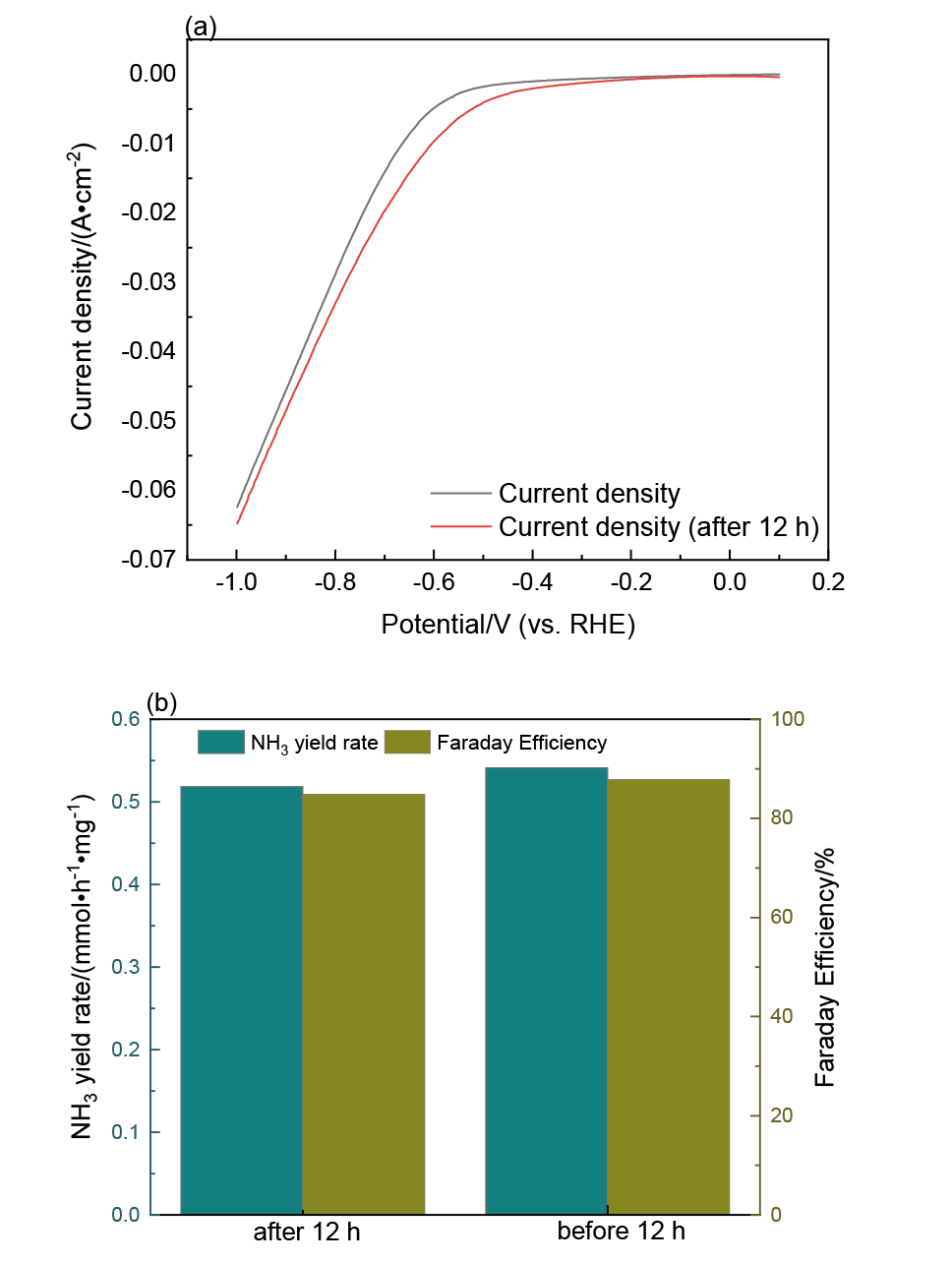
| [1] |
Lee, W. S.; Zhou, K. J.; Hepting, M. Nat. Phys. 2020, 17, 53.
doi: 10.1038/s41567-020-0993-7 |
| [2] |
Liu, H.; Lang, X.; Zhu, C. Angew. Chem. Int. Ed. 2022, 61, e202202556.
|
| [3] |
Hu, Q.; Qin, Y.; Wang, X. CCS Chem. 2022, 4, 2053.
doi: 10.31635/ccschem.021.202101042 |
| [4] |
Wang, J. H.; Wang, S.; Tong, X. H. J. Shaanxi University Sci. Tec. 2017, 35, 34 (in Chinese).
|
|
(王家宏, 王思, 童新豪, 陕西科技大学学报, 2017, 35, 34.)
|
|
| [5] |
MacFarlane, D. R.; Cherepanov, P. V.; Choi, J. Joule 2020, 4, 1186.
doi: 10.1016/j.joule.2020.04.004 |
| [6] |
Clark, C. A.; Reddy, C. P.; Xu, H. ACS Catal. 2019, 10, 494.
doi: 10.1021/acscatal.9b03239 |
| [7] |
Li, M. M.S. Thesis, China University of Petroleum (East China), Qingdao, 2014 (in Chinese).
|
|
(李猛, 硕士论文, 中国石油大学(华东), 青岛, 2014.)
|
|
| [8] |
Gu, L.; Kuang, M.; Chen, J. Chinese J. Struc. Chem. 2023, 42, 100067.
|
| [9] |
Wu, Z. Y.; Song, Y. H.; Liu, Y. B. Chem. Catalysis 2023, 3, 100786.
doi: 10.1016/j.checat.2023.100786 |
| [10] |
Hong, Q. L.; Zhou, J.; Zhai, Q. G. Chem. Commun. 2021, 57, 11621.
doi: 10.1039/D1CC04952F |
| [11] |
Cheng, J.; Sun, W.; Dai, G. Fuel 2023, 332.
|
| [12] |
Xu, D.; Li, Y.; Yin, L. Front. Environ. Sci. Eng. 2018, 12, 9.
|
| [13] |
Theerthagiri, J.; Park, J.; Das, H. T. Environ. Chem. Lett. 2022, 20, 2929.
doi: 10.1007/s10311-022-01469-y |
| [14] |
Dai, C. C.; Sun, Y. M.; Chen, G. Angew. Chem. Int. Ed. 2020, 59, 9418.
doi: 10.1002/anie.v59.24 |
| [15] |
Long, J.; Chen, S. M.; Zhang, Y. L. Angew. Chem. Int. Ed. 2020, 59, 9711.
doi: 10.1002/anie.202002337 pmid: 32189423 |
| [16] |
Chen, D.; Zhang, S. C.; Bu, X. M. Nano Energy 2022, 98.
|
| [17] |
Niu, Z. D.; Fan, S. Y.; Li, X. Y. ACS Appl. Energy Mater. 2022, 5, 3339.
doi: 10.1021/acsaem.1c03969 |
| [18] |
Chen, G. F.; Yuan, Y. F.; Jiang, H. F. Nat. Energy. 2020, 5, 605.
doi: 10.1038/s41560-020-0654-1 |
| [19] |
Dima, G. E.; Beltramo, G. L.; Koper, M. T. M. Electrochim. Acta 2005, 50, 4318.
doi: 10.1016/j.electacta.2005.02.093 |
| [20] |
Zhao, H.; Wang, F. D.; Qian, G. L. Ind. Water Treat. 2024, 44, 60 (in Chinese).
|
|
(赵慧, 王富东, 钱光磊, 工业水处理, 2024, 44, 60.)
doi: 10.19965/j.cnki.iwt.2022-1075 |
|
| [21] |
Reyter, D.; Chamoulaud, G.; Bélanger, D. J. Electroanal. Chem. 2006, 596, 13.
doi: 10.1016/j.jelechem.2006.06.012 |
| [22] |
Fu, X. B.; Zhao, X. G.; Hu, X. B. Appl. Mater. Today. 2020, 19.
|
| [23] |
Wang, X. D.; Zhu, M. Q.; Zeng, G. S. Nanoscale 2020, 12, 9385.
doi: 10.1039/C9NR10743F |
| [24] |
Sun, J.; Alam, D.; Daiyan, R. Energy Environ. Sci. 2021, 14, 865.
doi: 10.1039/D0EE03769A |
| [25] |
Wang, Y. T.; Zhou, W.; Jia, R. R. Angew. Chem. Int. Ed. 2020, 59, 5350.
doi: 10.1002/anie.v59.13 |
| [26] |
Zou, Y. J.; Xiao, K.; Qin, Q. ACS Nano 2021, 15, 6551.
doi: 10.1021/acsnano.0c09661 |
| [27] |
Liu, T. Y.; Yang, G. J.; Wang, W. Environ. Res. 2020, 188, 109741.
doi: 10.1016/j.envres.2020.109741 |
| [28] |
Li, K.; Cai, W.; Zhang, Z. C. Chem. Eng. J. 2022, 435, 135017.
doi: 10.1016/j.cej.2022.135017 |
| [29] |
Bai, J. L.; Yang, B. S.; Liu, B. Shanxi Daxue Xuebao, Ziran Kexueban 2022, 45, 1319 (in Chinese).
|
|
(白惊雷, 杨斌盛, 刘斌, 山西大学学报(自然科学版), 2022, 45, 1319.)
|
|
| [30] |
Ma, C.; Yu, Z. G.;, J. Wei, J. J.. Appl. Catal. B 2022, 319.
|
| [31] |
Nam, K. B.; Lee, S. H.; Hong, S. C. Appl. Surf. Sci. 2021, 544.
|
| [32] |
Feng, W. H, ; Fang, J. Z.; Zhou, G. Y. Mol. Catal. 2017, 434, 69.
|
| [33] |
Li, X. W.; Wang, B.; Yin, W. X. Acta Phys.-Chim. Sin. 2020, 36, 1902001.
doi: 10.3866/PKU.WHXB201902001 |
| [34] |
Wang, B. M.S. Thesis, Shanxi University, Taiyuan, 2021 (in Chinese).
|
|
(王波, 杨斌盛, 刘斌, 硕士论文, 山西大学, 太原, 2021.)
|
|
| [35] |
Song, T. L.; Zou, M. S.; Lu, D. F. Textbook of X-ray Photoelectron Spectroscopy Data Analysis, Beijing Institute of Technology Press, Beijing, 2022, p. 468 (in Chinese).
|
|
(宋廷鲁, 邹美帅, 鲁德凤, X射线光电子能谱数据分析, 北京理工大学出版社, 北京(自然科学版), 2022, p. 468.)
|
|
| [36] |
Yu, K. Q. M.S. Thesis, Jilin University, Changchun, 2023 (in Chinese).
|
|
(于凯强, 硕士论文, 吉林大学, 长春(自然科学版), 2023.)
|
|
| [37] |
Lin, Y. X.; Zhang, S. N.; Xue, Z. H. Nat. Commun. 2019, 10, 4380.
doi: 10.1038/s41467-019-12312-4 |
| [38] |
Liu, S. Y. M.S. Thesis, Inner Mongolia University, Hohhot, 2022 (in Chinese).
|
|
(刘思媛, 硕士论文, 内蒙古大学, 呼和浩特, 2022.)
|
| [1] | Chunmeng Li, Zhe Bi, Haichao Wang, Keding Lu. Field Measurement of Alkyl Nitrates in the Atmosphere [J]. Acta Chimica Sinica, 2024, 82(3): 323-335. |
| [2] | Lü Xijuan, Zhang Yunhong. Volatility of Ammonium Nitrate in Ultra-viscous Aerosol Droplets by Optical Tweezers [J]. Acta Chimica Sinica, 2020, 78(4): 326-329. |
| [3] | Wang Chen, Chen Rui, Song Lin, Zhang Naidong. Characteristics of Some Typical Inorganic Oxyacid Free Radicals [J]. Acta Chim. Sinica, 2019, 77(3): 205-212. |
| [4] | Li Yanxia, Duan Xiaoyong, Li Xianguo, Tang Xuli. Mechanism Study on Photodegradation of Nonylphenol in Water by Intermediate Products Analysis [J]. Acta Chimica Sinica, 2012, 70(17): 1819-1826. |
| [5] | Wang Qing, Shang Jing, Song Han. Photoelectrocatalytic Reduction of Cr(VI) over TiO2 Nanotube Arrays under Half-wave Pulsed Direct Current [J]. Acta Chimica Sinica, 2012, 0(04): 405-410. |
| [6] | Hu Jingfang, Sun Jizhou, Bian Chao, Tong Jianhuaa, Li Yang, Xia Shanhong. Study on Micro-sensing Chip of Nitrate Based on Three-dimensional Nano-structured Silver Modified Electrode [J]. Acta Chimica Sinica, 2012, 70(03): 291-296. |
| [7] | Lü Shaoyi, Shao Ziqiang, Zhang Zhenling, Wang Huiqing, Wang Wenjun. Studies on Rheological Properties of Gelled Propellant Based on New Energetic Cellulose [J]. Acta Chimica Sinica, 2012, 70(02): 200-206. |
| [8] | CHEN Xiao-Wei, ZHAN Zu-Jin, LI Pu-Rui, GE Zhong-Xue, LEI Ming. Kinetics Study of the Synthesis of Butyl(2-azidoethyl)nitramine from the Reaction of 2-[Butyl(nitro)amino]ethyl nitrate with Sodium Azide [J]. Acta Chimica Sinica, 2011, 69(20): 2523-2526. |
| [9] | ZOU Ben-Xue, BIAN Li-Jun, WANG Yan, LI Xin-Jie, LIU Xiao-Xia. Electrocatalytic Reduction of Bromate, Chlorate, Nitrite and 4-Nitrophenol at WO3/PANI Modified Electrode [J]. Acta Chimica Sinica, 2011, 69(13): 1575-1581. |
| [10] | Huang Ke-Jing. Sensitive Determination of Nitrate and Nitrite in Food Products by Spectrofluorimetry with a Fluorescent Probe: 8-(3’,4’- Diaminophenyl)-difluoroboradiaza-s-indacence [J]. Acta Chimica Sinica, 2009, 67(10): 1075-1080. |
| [11] | XIA Lu1,2 XIAO Ji-Jun2 FAN Jian-Fen ZHU Wei2 XIAO He-Ming*,2. Molecular Dynamics Simulation of Mechanical Properties and Surface Interaction for Nitrate Plasticizer [J]. Acta Chimica Sinica, 2008, 66(8): 874-878. |
| [12] | . Effect of the Different Compound Systems on Photocatalytic Reduction of Cr(VI) by Titanium-Bearing Blast Furnace Slag [J]. Acta Chimica Sinica, 2008, 66(22): 2539-2546. |
| [13] | LIU, Chun-Li *,a MA, Lin b LIN, Rui-Sen c. Volumetric Properties of Glycine and L-Serine in Aqueous LiNO3, NaNO3 and KNO3 Solutions at 298.15 K [J]. Acta Chimica Sinica, 2008, 66(14): 1632-1636. |
| [14] |
HU, Xue-Lei *,a,b CHEN, Zhong a QIU, Lia LIU, Bo b ZHAO, Yuan-Di b PAN, Zhi-Quan a LUO, Qin-Hui c . Synthesis, Crystal Structure and Luminescent Property of a Europium Nitrate Ternary Complex with a Diphenol Macrocyclic Ligand [J]. Acta Chimica Sinica, 2008, 66(12): 1446-1450. |
| [15] | LIU Guo-Sheng*,1,2; RAN Zhi-Lin; WANG Hai-Lei; LIU Yi*,2; SHEN Ping3; LU Yan4. Study on the Eruption of Heat for Escherichia coli B Aroused by Lanthanum Nitrate and Its Mechanism [J]. Acta Chimica Sinica, 2007, 65(10): 917-922. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||