Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (11): 1114-1119.DOI: 10.6023/A24090273 Previous Articles Next Articles
Communication
投稿日期:2024-09-11
发布日期:2024-10-16
基金资助:Received:2024-09-11
Published:2024-10-16
Contact:
*E-mail: gaopan@yzu.edu.cn
Supported by:Share
Yuhan Liu, Pan Gao. Direct Borylation of Organohalides Using Mechanochemically Generated Calcium-Based Heavy Grignard Reagents (R-CaX)[J]. Acta Chimica Sinica, 2024, 82(11): 1114-1119.
| Entry | Various of standard conditions | Yield of 3ab/% |
|---|---|---|
| 1 | none | 85 |
| 2 | 3.0 equiv. of THF | 78 |
| 3 | 5.0 equiv. of THF | 60 |
| 4 | DME instead of THF | 66 |
| 5 | 1,4-dioxone instead of THF | 45 |
| 6 | Et2O instead of THF | 71 |
| 7 | DMF instead of THF | 12 |
| 8 | toluene instead of THF | 6 |
| 9 | hexane instead of THF | 19 |
| 10 | 1.0 equiv. of Ca | 69 |
| 11 | 2.0 equiv. of Ca | 77 |
| 12 | 4.0 equiv. of 2 | 80 |
| 13 | 2.0 equiv. of 2 | 55 |
| 14 | frequency=15 Hz | 45 |
| 15 | 80 ℃ | 84 |
| 16 | Zn instead of Ca | 21 |
| 17 | Mg instead of Ca | 49 |
| 18 | Li instead of Ca | 33 |
| 19 | under N2 | 81 |
| 20 | in solutionc | 0 |
| Entry | Various of standard conditions | Yield of 3ab/% |
|---|---|---|
| 1 | none | 85 |
| 2 | 3.0 equiv. of THF | 78 |
| 3 | 5.0 equiv. of THF | 60 |
| 4 | DME instead of THF | 66 |
| 5 | 1,4-dioxone instead of THF | 45 |
| 6 | Et2O instead of THF | 71 |
| 7 | DMF instead of THF | 12 |
| 8 | toluene instead of THF | 6 |
| 9 | hexane instead of THF | 19 |
| 10 | 1.0 equiv. of Ca | 69 |
| 11 | 2.0 equiv. of Ca | 77 |
| 12 | 4.0 equiv. of 2 | 80 |
| 13 | 2.0 equiv. of 2 | 55 |
| 14 | frequency=15 Hz | 45 |
| 15 | 80 ℃ | 84 |
| 16 | Zn instead of Ca | 21 |
| 17 | Mg instead of Ca | 49 |
| 18 | Li instead of Ca | 33 |
| 19 | under N2 | 81 |
| 20 | in solutionc | 0 |
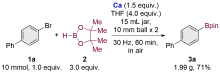
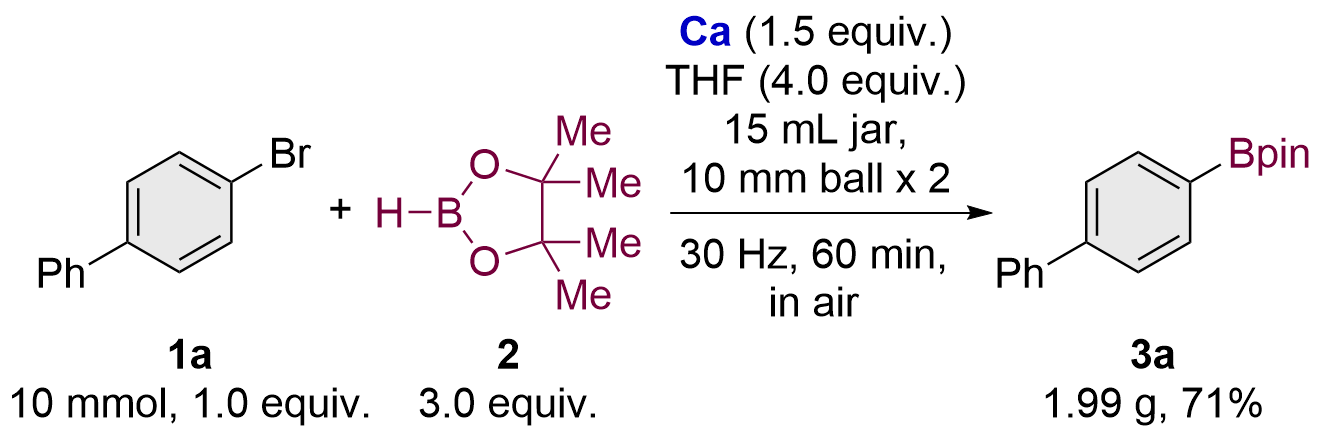
| [1] |
Hanusa, T. P. Chem. Rev. 1993, 93, 1023.
|
| [2] |
The Chemistry of Organo-magnesium Compounds, Eds.: Rappoport, Z.; Marek, I., Wiley-VCH, Weinheim, Germany, 2008.
|
| [3] |
(a) Banno, T.; Hayakawa, Y.; Umeno, M. J. Organomet. Chem. 2002, 653, 288.
|
|
(b) Peltzer, R. M.; Gauss, J.; Eisenstein, O.; Cascella, M. J. Am. Chem. Soc. 2020, 142, 2984.
|
|
| [4] |
Harder, S. Chem. Rev. 2010, 110, 3852.
|
| [5] |
Holleman, A. F.; Wiberg, E.; Wiberg, N. Inorganic Chemistry, De Gruyter, Berlin, 2001.
|
| [6] |
(a) Wu, T.-C.; Xiong, H.; Rieke, R. D. J. Org. Chem 1990, 55, 5045.
pmid: 27976821 |
|
(b) Mochida, K.; Ogawa, H. J. Organomet. Chem. 1983, 243, 131.
pmid: 27976821 |
|
|
(c) Li, H.; Wang, X.-Y.; Wei, B.; Xu, L.; Zhang, W.-X.; Pei, J.; Xi, Z. Nat. Commun. 2014, 5, 4508.
pmid: 27976821 |
|
|
(d) Westerhausen, M.; Koch, A.; Görls, H.; Krieck, S. Chem.-Eur. J. 2017, 23, 1456.
doi: 10.1002/chem.201603786 pmid: 27976821 |
|
| [7] |
Beckmann, E. Ber. Dtsch. Chem. Ges 1905, 38, 904.
|
| [8] |
(a) Gilman, H.; Schulze, F. J. Am. Chem. Soc 1926, 48, 2463.
|
|
(b) Bryce-Smith, D.; Skinner, A. C. J. Chem. Soc 1963, 577.
|
|
|
(c) Kawabata, N.; Matsumura, A.; Yamashita, S. Tetrahedron 1973, 29, 1069.
|
|
|
(d) Kawabata, N.; Matsumura, A.; Yamashita, S. J. Org. Chem 1973, 38, 4268.
|
|
| [9] |
(a) Langer, J.; Krieck, S.; Fischer, R.; Görls, H.; Walther, D.; Westerhausen, M. Organometallics 2009, 28, 5814.
|
|
(b) Maercker, A. Angew. Chem. Int. Ed. 1987, 26, 972.
|
|
| [10] |
Westerhausen, M.; Langer, J.; Krieck, S.; Fischer, R.; Görls, H.; Köhler, M. Top. Organomet. Chem 2013, 45, 29.
|
| [11] |
D'Alterio, M. C.; Casals-Cruañas, È.; Tzouras, N. V.; Talarico, G.; Nolan, S. P.; Poater, A. Chem. Eur. J 2021, 27, 13481.
|
| [12] |
Baker, R. H.; Schlesinger, A. H. J. Am. Chem. Soc 1945, 67, 1499.
|
| [13] |
Chen, J.-Q.; Li, J.-H.; Dong, Z.-B. Adv. Synth. Catal 2020, 362, 3311.
|
| [14] |
(a) Zhang, Z.; Huang, S.; Liu, W.; Zhao, L.-L.; Hu, C.; Yan, X. Green Synth. Catal 2023, 4, 300.
|
|
(b) McCallum, T. Green Synth. Catal 2023, 4, 10.
|
|
|
(c) Wang, H.; Xu, T. Chinese J. Org. Chem 2023, 43, 3328 (in Chinese).
|
|
|
(王贺盼, 徐涛, 有机化学, 2023, 43, 3328.)
|
|
| [15] |
(a) James, S. L.; Adams, C. J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K. D. M.; Hyett, G.; Jones, V.; Krebs, A.; Mack, J.; Maini, L.; Orpen, A. G.; Parkin, I. P.; Shearouse, W. C.; Steed, J. W.; Waddell, D. C. Chem. Soc. Rev 2012, 41, 413.
|
|
(b) Zhou, K.; Mao, Y.; Wu, F.; Lou, S.; Xu, D. Chinese J. Org. Chem. 2021, 41, 4623 (in Chinese).
doi: 10.6023/cjoc202106046 |
|
|
(周琨, 毛羊杰, 吴峰伟, 娄绍杰, 许丹倩, 有机化学, 2021, 41, 4623.)
|
|
|
(c) Wang, H.; Ying, P.; Yu, J.; Su, W. Chinese J. Org. Chem. 2021, 41, 1897 (in Chinese).
doi: 10.6023/cjoc202009053 |
|
|
(王浩, 应娉, 俞静波, 苏为科, 有机化学, 2021, 41, 1897.)
|
|
| [16] |
(a) Wang, G.-W.; Komatsu, K.; Murata, Y.; Shiro, M. Nature 1997, 387, 583.
|
|
(b) Wang, G.-W. Chin. J. Chem 2021, 39, 1797.
|
|
|
(c) Li, L.; Niu, C.; Wang, G.-W. Chin. J. Chem 2022, 40, 2539.
|
|
| [17] |
Koch, A.; Dufrois, Q.; Wirgenings, M.; Görls, H.; Krieck, S.; Etienne, M.; Pohnert, G.; Westerhausen, M. Chem.-Eur. J. 2018, 24, 16840.
|
| [18] |
Pharmacopeia of the United States of America, 32nd revision, and the National Formulary, 27th ed., US Pharmacopeia, 2009.
|
| [1] | Abuduwaili Kadierya, Yunusi Reziguli, Jiajia Li, Shiwei Luo, Abudu Rexit Abulikemu. Solvent-free Oxidative Dehydrogenation N—N Coupling Reaction of N-Alkoxyamides [J]. Acta Chimica Sinica, 2024, 82(7): 731-735. |
| [2] | Chuwen Luo, Chaoying Kong, Zhaohui Tang. Research Progress in Sonochemistry for Biomedical Applications★ [J]. Acta Chimica Sinica, 2023, 81(7): 836-842. |
| [3] | Li Liu, Gang Zheng, Guoqiang Fan, Hongguang Du, Jiajing Tan. Research Progress in Organic Reactions Involving 4-Acyl/Carbamoyl/Alkoxycarbonyl Substituted Hantzsch Esters [J]. Acta Chimica Sinica, 2023, 81(6): 657-668. |
| [4] | Jingpeng Li, Qi Yang, Zhou Zhang, Guiyun Zeng, Teng Liu, Chao Huang. Highly Selective Synthesis of (Z)-N-vinyl Ring N,O-Acetal Derivatives by Multi-component Continuous Flow [J]. Acta Chimica Sinica, 2022, 80(11): 1463-1468. |
| [5] | Wenjun Wu, Yuting Li, Xi Feng, Wenxing Ding. Perovskite Dual-function Passivator: Room Temperature Ionic Liquid Obtained from Mechanochemical Preparation [J]. Acta Chimica Sinica, 2022, 80(11): 1469-1475. |
| [6] | Wei Zheyu, Chang Yalin, Yu Han, Han Sheng, Wei Yongge. Application of Anderson Type Heteropoly Acids as Catalysts in Organic Synthesis [J]. Acta Chimica Sinica, 2020, 78(8): 725-732. |
| [7] | Dong Kui, Liu Qiang, Wu Li-Zhu. Cross-Coupling Hydrogen Evolution Reactions [J]. Acta Chimica Sinica, 2020, 78(4): 299-310. |
| [8] | Liu Qianyi, Zhang Lei, Mo Fanyang. Organic Borylation Reactions via Radical Mechanism [J]. Acta Chimica Sinica, 2020, 78(12): 1297-1308. |
| [9] | Ye Wenbo, Yan Zicong, Wan Changfeng, Hou Haoqing, Wang Zhiyong. A New Decarboxylation/Methylation Process of Cinnamic Acids [J]. Acta Chim. Sinica, 2018, 76(2): 99-102. |
| [10] | Pei Pengkun, Zhang Fan, Yi Hong, Lei Aiwen. Visible Light Promoted Benzylic Csp3-H Bond Activation and Functionalization [J]. Acta Chim. Sinica, 2017, 75(1): 15-21. |
| [11] | Zhou Baolong, Chen Long. New Strategies for the Synthesis of Covalent Organic Porous Polymers [J]. Acta Chim. Sinica, 2015, 73(6): 487-497. |
| [12] | Lu Qingquan, Yi Hong, Lei Aiwen. Autoxidative Coupling and Its Applications to C-H Functionalization [J]. Acta Chim. Sinica, 2015, 73(12): 1245-1249. |
| [13] | LOU Feng-Wen, ZHOU Jian-Feng, LIN Xian-Fu. Controllable Anti-Markovnikov and Markovnikov Addition of Thiols to Vinyl Ethers for the Synthesis of Alkyloxy Thioethers [J]. Acta Chimica Sinica, 2010, 68(12): 1223-1228. |
| [14] | CAO Jie-Ming*,ZHENG Ming-Bo,LU Peng,DENG Shao-Gao,CHEN Yong-Ping,WEN Fan,GUO Jing,ZHANG Fang,TAO Jie. Synthesis of Silver Nanoparticles by Reductive Polysaccharides [J]. Acta Chimica Sinica, 2005, 63(16): 1541-1544. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
