Acta Chimica Sinica ›› 2025, Vol. 83 ›› Issue (10): 1174-1183.DOI: 10.6023/A25060209 Previous Articles Next Articles
Article
投稿日期:2025-06-09
发布日期:2025-08-21
通讯作者:
高希珂
基金资助:
Diefeng Rena,b, Junjun Xiangb, Xike Gaob,*( )
)
Received:2025-06-09
Published:2025-08-21
Contact:
Xike Gao
Supported by:Share
Diefeng Ren, Junjun Xiang, Xike Gao. Design, Synthesis, and Anti-inflammatory/Antioxidant Activities of Guaiazulene Dimers Bridged by Triethylene Glycol[J]. Acta Chimica Sinica, 2025, 83(10): 1174-1183.
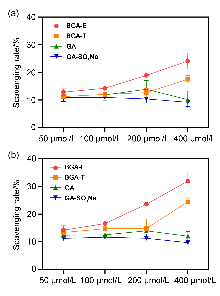

| 化合物 | 时间/h | 自由基清除率a/% | |||
|---|---|---|---|---|---|
| 50 μmol•L−1 | 100 μmol•L−1 | 200 μmol•L−1 | 400 μmol•L−1 | ||
| BGA-E | 0.5 | 12.86 | 14.25 | 18.90 | 24.13 |
| 4 | 14.19 | 16.58 | 23.58 | 31.91 | |
| BGA-T | 0.5 | 11.25 | 12.06 | 12.70 | 17.52 |
| 4 | 13.35 | 14.72 | 14.83 | 24.44 | |
| GA | 0.5 | 11.84 | 11.86 | 13.88 | 10.15 |
| 4 | 12.35 | 12.51 | 13.91 | 12.03 | |
| GA-SO3Na | 0.5 | 10.86 | 10.95 | 10.40 | 9.20 |
| 4 | 11.23 | 11.64 | 11.24 | 9.69 | |
| 化合物 | 时间/h | 自由基清除率a/% | |||
|---|---|---|---|---|---|
| 50 μmol•L−1 | 100 μmol•L−1 | 200 μmol•L−1 | 400 μmol•L−1 | ||
| BGA-E | 0.5 | 12.86 | 14.25 | 18.90 | 24.13 |
| 4 | 14.19 | 16.58 | 23.58 | 31.91 | |
| BGA-T | 0.5 | 11.25 | 12.06 | 12.70 | 17.52 |
| 4 | 13.35 | 14.72 | 14.83 | 24.44 | |
| GA | 0.5 | 11.84 | 11.86 | 13.88 | 10.15 |
| 4 | 12.35 | 12.51 | 13.91 | 12.03 | |
| GA-SO3Na | 0.5 | 10.86 | 10.95 | 10.40 | 9.20 |
| 4 | 11.23 | 11.64 | 11.24 | 9.69 | |
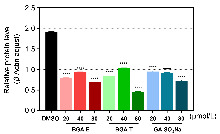
| 化合物 | OD值 | |||
|---|---|---|---|---|
| 50 μmol•L−1 | 100 μmol•L−1 | 200 μmol•L−1 | 400 μmol•L−1 | |
| BGA-E | 0.0685 | 0.0827 | 0.1011 | 0.1389 |
| BGA-T | 0.1005 | 0.1503 | 0.2267 | 0.3357 |
| GA | 0.1084 | 0.1772 | 0.3135 | 0.5741 |
| GA-SO3Na | 0.0955 | 0.1370 | 0.2090 | 0.3346 |
| 化合物 | OD值 | |||
|---|---|---|---|---|
| 50 μmol•L−1 | 100 μmol•L−1 | 200 μmol•L−1 | 400 μmol•L−1 | |
| BGA-E | 0.0685 | 0.0827 | 0.1011 | 0.1389 |
| BGA-T | 0.1005 | 0.1503 | 0.2267 | 0.3357 |
| GA | 0.1084 | 0.1772 | 0.3135 | 0.5741 |
| GA-SO3Na | 0.0955 | 0.1370 | 0.2090 | 0.3346 |
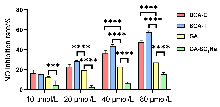

| 化合物 | 浓度/(μmol•L−1) | 抑制率a/% |
|---|---|---|
| BGA-E | 10 | 16.60 |
| 20 | 23.01 | |
| 40 | 36.23 | |
| 80 | 47.75 | |
| BGA-T | 10 | 15.61 |
| 20 | 28.57 | |
| 40 | 43.99 | |
| 80 | 57.92 | |
| GA | 10 | 12.88 |
| 20 | 19.52 | |
| 40 | 23.05 | |
| 80 | 27.19 | |
| GA-SO3Na | 10 | 4.49 |
| 20 | 2.85 | |
| 40 | 6.18 | |
| 80 | 15.32 | |
| 模型组 | — | 0 |
| 化合物 | 浓度/(μmol•L−1) | 抑制率a/% |
|---|---|---|
| BGA-E | 10 | 16.60 |
| 20 | 23.01 | |
| 40 | 36.23 | |
| 80 | 47.75 | |
| BGA-T | 10 | 15.61 |
| 20 | 28.57 | |
| 40 | 43.99 | |
| 80 | 57.92 | |
| GA | 10 | 12.88 |
| 20 | 19.52 | |
| 40 | 23.05 | |
| 80 | 27.19 | |
| GA-SO3Na | 10 | 4.49 |
| 20 | 2.85 | |
| 40 | 6.18 | |
| 80 | 15.32 | |
| 模型组 | — | 0 |
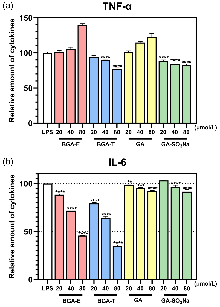

| 化合物 | 浓度/ (μmol•L−1) | 相对含量a/% | |
|---|---|---|---|
| TNF-α | IL-6 | ||
| BGA-E | 20 | 101.71 | 88.40 |
| 40 | 105.33 | 71.76 | |
| 80 | 139.79 | 45.83 | |
| BGA-T | 20 | 94.47 | 79.25 |
| 40 | 89.67 | 63.89 | |
| 80 | 77.14 | 34.89 | |
| GA | 20 | 101.29 | 98.72 |
| 40 | 114.84 | 95.28 | |
| 80 | 122.68 | 92.17 | |
| GA-SO3Na | 20 | 88.96 | 103.13 |
| 40 | 84.68 | 96.59 | |
| 80 | 82.98 | 91.48 | |
| 模型组 | — | 100.00 | 100.00 |
| 化合物 | 浓度/ (μmol•L−1) | 相对含量a/% | |
|---|---|---|---|
| TNF-α | IL-6 | ||
| BGA-E | 20 | 101.71 | 88.40 |
| 40 | 105.33 | 71.76 | |
| 80 | 139.79 | 45.83 | |
| BGA-T | 20 | 94.47 | 79.25 |
| 40 | 89.67 | 63.89 | |
| 80 | 77.14 | 34.89 | |
| GA | 20 | 101.29 | 98.72 |
| 40 | 114.84 | 95.28 | |
| 80 | 122.68 | 92.17 | |
| GA-SO3Na | 20 | 88.96 | 103.13 |
| 40 | 84.68 | 96.59 | |
| 80 | 82.98 | 91.48 | |
| 模型组 | — | 100.00 | 100.00 |

| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
doi: 10.1021/acs.jmedchem.5b01380 pmid: 27010926 |
| [9] |
|
| [10] |
pmid: 9306266 |
| [11] |
pmid: 12049221 |
| [12] |
|
| [13] |
((a)
|
|
(李蕾, 朱聪聪, 朱权刚, 陈中建, 高希珂, 有机化学, 2022, 42, 2906.)
doi: 10.6023/cjoc202204025 |
|
|
((b)
|
|
|
肖梦佳, 高希珂, 有机化学, 2023, 43, 3246.).
|
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
|
(张晓雨, 李欣燕, 崔冰, 邵志晖, 赵铭钦, 有机化学, 2023, 43, 2885.)
doi: 10.6023/cjoc202301007 |
|
| [21] |
(a)
|
|
((b)
|
|
|
(李瑶, 陈丙年, 罗丹, 雷珊, 王力, 化学学报, 2023, 81, 1318.)
doi: 10.6023/A23060276 |
|
| [22] |
doi: 10.1006/abio.1996.0292 pmid: 8660627 |
| [23] |
|
| [24] |
doi: 10.1016/0092-8674(78)90101-0 pmid: 212198 |
| [25] |
|
| [26] |
doi: 10.1111/j.1365-2591.2012.02096.x pmid: 22788664 |
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [1] | Yinghui Wang, Yuhui Wang, Jiayao Xiong, Siqi Su, Mengke Hao, Simin Wei. The Fabrication of Silver Nanoparticles Using Puerarin and the Photothermal Sterilization and Diabetic Wound Healing Behavior [J]. Acta Chimica Sinica, 2024, 82(11): 1150-1161. |
| [2] | Xueyang Yin, Kai Gu, Zhengzhong Shao. Preparation of the Protein/Polyphenylboronic Acid Nanospheres for Drug Loading and Unloading [J]. Acta Chimica Sinica, 2023, 81(2): 116-123. |
| [3] | Jinyuan Zhao, Qian Zhang, Jian Wang, Qi Zhang, Heng Li, Yaping Du. Advances in the Scavenging Materials for Reactive Oxygen Species [J]. Acta Chimica Sinica, 2022, 80(4): 570-580. |
| [4] | Zhou Yiqing, Xiao Youli. Target Identification of Bioactive Natural Products [J]. Acta Chim. Sinica, 2018, 76(3): 177-189. |
| [5] | XIE Hu-Jun, LEI Qun-Fang, FANG Wen-Jun. Density Functional Theory Study on the Antioxidation Activity of Quercetin [J]. Acta Chimica Sinica, 2010, 68(15): 1467-1472. |
| [6] | xiaoqun wang. The Quantitative Analysis and Actually Processing Addition Determination of Antioxidant and Nucleating Agent in PP [J]. Acta Chimica Sinica, 2010, 68(04): 358-362. |
| [7] | . Synthesis of 3β-Hydroxy-24-norchol-5-en-23-oic Acid [J]. Acta Chimica Sinica, 2009, 67(14): 1700-1704. |
| [8] | YU Qin NAN Feng XIANG Jin LIANG Mao-Zhi* QIN Yong-Ping. Retention Characteristics and Separation Mechanism of Enantiomers of Arylpropionic Acid Nonsteroidal Antiinflammatory Drugs with OJ Column [J]. Acta Chimica Sinica, 2008, 66(9): 1079-1085. |
| [9] | . Synthesis and Spectrum Stability of an Oligomeric Terfluorene Tethered with Antioxidant Hindered Amine [J]. Acta Chimica Sinica, 2008, 26(23): 2575-2578. |
| [10] | YAN Xi* XIA Ling-Ling YU Jing DING Wan-Jian . Synthesis, and Studies on Antioxidation Properties of two 4’-p-toluenesulfonylisoflavone Derivatives [J]. Acta Chimica Sinica, 2008, 66(1): 39-43. |
| [11] | YANG Chang-Ying1,2; LIU Yi*,1,3; ZENG Fang2; LI Jing2; LI Qiang-Guo; LI Lin-Wei. Interaction of Three Kinds of Non-steroidal Anti-inflammatory Drugs with DNA Investigated by Two Fluorescence Probes [J]. Acta Chimica Sinica, 2007, 65(18): 2076-2080. |
| [12] | YAN Xi*; LI Yu-Mei; YU Jing; DING Wan-Jian. Synthesis and Antioxidant Activities of Two New Water Soluble Isoflavone Derivatives [J]. Acta Chimica Sinica, 2007, 65(17): 1845-1850. |
| [13] | WANG Jian-Guo*,1; JIANG Li-Li; YANG Wei-Wei; CHI Hong-Xun2; SUN Qiao-Hua; LÜ Hui. Electrochemical Study on the Destructive Effect of Hydroxyl Free Radical on Supported Bilayer Lipid Membrane and Protective Effect of Water-Soluble Antioxidant [J]. Acta Chimica Sinica, 2007, 65(13): 1234-1238. |
| [14] | YAN Xi*, LIU Yun, LI Jia-Mian, YAN Hui-Qing, ZHENG Ze-Bao, SI Shu-Feng. Synthesis, Crystal Structure and Properties of Cobalt(II) 2,4,4'-Trihydroxydeoxybenzoin-3'-sulfonate [J]. Acta Chimica Sinica, 2006, 64(9): 935-942. |
| [15] | SHI Yan, ZHAN Xian-Cheng*, LÜ Tai-Ping, LI Lin, CAO Cheng-Yong, SHU Xiao-Ming, LI Cheng-Rong, LI Lin-Li. Determination of Oxidation Rate Constants of Antioxidants Sodium Sulfite, Sodium Bisulfite and Sodium Pyrosulfite [J]. Acta Chimica Sinica, 2006, 64(6): 496-500. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||