Acta Chimica Sinica ›› 2025, Vol. 83 ›› Issue (11): 1372-1378.DOI: 10.6023/A25060238 Previous Articles Next Articles
Article
苏秦a, 雷平a, 王栋a, Shahid Ali Khanb, 阿布拉江•克依木a,*( )
)
投稿日期:2025-06-26
发布日期:2025-08-11
通讯作者:
阿布拉江?克依木
基金资助:
Su Qina, Lei Pinga, Wang Donga, Shahid Ali Khanb, Ablajan Keyumea,*( )
)
Received:2025-06-26
Published:2025-08-11
Contact:
Ablajan Keyume
Supported by:Share
Su Qin, Lei Ping, Wang Dong, Shahid Ali Khan, Ablajan Keyume. Visible-Light-Induced Defluorinative Acylation of α-Trifluoromethyl Alkenes with Aromatic Carboxylic Anhydrides[J]. Acta Chimica Sinica, 2025, 83(11): 1372-1378.
| Entry | Variations from standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 82 |
| 2 | Ir[dF(CF3)ppy]2(dtbbpy)PF6 or Eosin Y was used | 43, 16 |
| 3 | Ru(bpy)3Cl2 was used | N.R. |
| 4 | DBU or DIPEA was used | 46, 50 |
| 5 | NaHCO3 or K2CO3 was used | 36, 38 |
| 6 | EA, MeOH, DCE or DMSO was used | 34, Trace, 33, 30 |
| 7 | Addition 4 Å molecular sieves | 64 |
| 8 | No PC, or no Cs2CO3 | trace |
| 9 | No light, or no Ph3P | N.R. |
| 10 | Under air | N.R. |
| 11 | λ=390~395 nm | 69 |
| Entry | Variations from standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 82 |
| 2 | Ir[dF(CF3)ppy]2(dtbbpy)PF6 or Eosin Y was used | 43, 16 |
| 3 | Ru(bpy)3Cl2 was used | N.R. |
| 4 | DBU or DIPEA was used | 46, 50 |
| 5 | NaHCO3 or K2CO3 was used | 36, 38 |
| 6 | EA, MeOH, DCE or DMSO was used | 34, Trace, 33, 30 |
| 7 | Addition 4 Å molecular sieves | 64 |
| 8 | No PC, or no Cs2CO3 | trace |
| 9 | No light, or no Ph3P | N.R. |
| 10 | Under air | N.R. |
| 11 | λ=390~395 nm | 69 |
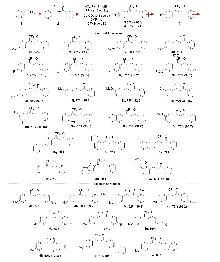

| [1] |
(a)
doi: 10.1002/ps.v80.7 |
|
(b)
doi: 10.1016/j.ecoenv.2024.117615 |
|
|
(c)
doi: 10.1016/j.cclet.2019.07.064 |
|
|
(d)
doi: 10.1021/acs.jafc.4c09728 |
|
|
(e)
|
|
|
(黄维垣, 钱昭辉, 化学学报, 1987, 45, 1175.)
|
|
|
(f)
doi: 10.6023/A2024E001 |
|
|
(胡金波, 化学学报, 2024, 82, 103.)
doi: 10.6023/A2024E001 |
|
| [2] |
(a)
doi: 10.1039/B610213C pmid: 26200936 |
|
(b)
doi: 10.1021/jm800219f pmid: 26200936 |
|
|
(c)
doi: 10.1021/acs.jmedchem.5b00258 pmid: 26200936 |
|
|
(d)
doi: 10.1021/acs.jmedchem.5b01455 pmid: 26200936 |
|
|
(e)
pmid: 26200936 |
|
|
(谷妍, 董喜城, 陈海峰, 陈敏伯, 张世相, 袁身刚, 郑崇直, 化学学报, 2000, 58, 1540.)
pmid: 26200936 |
|
| [3] |
(a)
doi: 10.1002/cjoc.v36.3 |
|
(b)
doi: 10.3390/app10196921 |
|
|
(c)
doi: 10.6023/A18080340 |
|
|
(金维则, 陆国林, 李永军, 黄晓宇, 化学学报, 2018, 76, 739.)
doi: 10.6023/A18080340 |
|
|
(d)
|
|
|
(朱万强, 唐国风, 勾华, 化学学报, 2007, 65, 1875.)
|
|
|
(e)
doi: 10.6023/A24060183 |
|
|
(王甦昊, 胡明霞, 陈卉, 赵彦英, 化学学报, 2024, 82, 925.)
doi: 10.6023/A24060183 |
|
|
(f)
doi: 10.6023/A23080387 |
|
|
(易敬霖, 陈茂, 化学学报, 2024, 82, 126.)
doi: 10.6023/A23080387 |
|
| [4] |
(a)
doi: 10.1021/cr100166a |
|
(b)
doi: 10.1021/cr500368h |
|
|
(c)
doi: 10.1021/ar500125m |
|
|
(d)
doi: 10.1002/anie.v60.21 |
|
| [5] |
(a) O’Hagan, D. Chem. Soc. Rev. 2008, 37, 308.
doi: 10.1039/B711844A |
|
(b)
doi: 10.1126/science.1215220 |
|
|
(c)
doi: 10.1039/C3CS60193E |
|
|
(d)
doi: 10.1021/jacs.7b00118 |
|
|
(e)
doi: 10.6023/A23040154 |
|
|
(秦沛, 马海, 张发光, 马军安, 化学学报, 2023, 81, 697.)
doi: 10.6023/A23040154 |
|
|
(f)
doi: 10.6023/cjoc202204060 |
|
|
(郭檬檬, 于子伦, 陈玉兰, 葛丹华, 马猛涛, 沈志良, 褚雪强, 有机化学, 2022, 42, 3562.)
doi: 10.6023/cjoc202204060 |
|
| [6] |
(a)
doi: 10.1021/ja021487+ |
|
(b)
doi: 10.1021/jm034162s |
|
|
(c)
doi: 10.1016/j.ejmech.2017.11.055 |
|
|
(d)
doi: 10.1016/j.jfluchem.2005.12.013 |
|
|
(e)
doi: 10.1021/ol017112v |
|
|
(f)
doi: 10.1021/ja00027a056 |
|
| [7] |
(a)
doi: 10.1021/acs.orglett.3c04094 pmid: 3039140 |
|
(b)
doi: 10.1021/jm900321u pmid: 3039140 |
|
|
(c)
pmid: 3039140 |
|
|
(d)
doi: 10.6023/A22110454 pmid: 3039140 |
|
|
(杨春晖, 陈景超, 李新汉, 孟丽, 王凯民, 孙蔚青, 樊保敏, 化学学报, 2023, 81, 1.)
doi: 10.6023/A22110454 pmid: 3039140 |
|
|
(e)
doi: 10.6023/cjoc202106013 pmid: 3039140 |
|
|
(李志清, 邱潇杨, 娄江, 王强, 有机化学, 2021, 41, 4192.)
doi: 10.6023/cjoc202106013 pmid: 3039140 |
|
|
(f)
doi: 10.6023/cjoc202000056 pmid: 3039140 |
|
|
(倪传法, 胡金波, 有机化学, 2020, 40, 2997.)
doi: 10.6023/cjoc202000056 pmid: 3039140 |
|
| [8] |
(a)
doi: 10.1021/jm1013693 |
|
(b)
doi: 10.1039/D0CC04318D |
|
|
(c)
doi: 10.1021/acs.orglett.0c00568 |
|
| [9] |
doi: 10.1021/acs.orglett.4c00112 |
| [10] |
doi: 10.1016/j.cclet.2023.109443 |
| [11] |
(a)
doi: 10.1039/D3QO01551C |
|
(b)
doi: 10.6023/A23080395 |
|
|
(张大伟, 赵海洋, 冯笑甜, 顾玉诚, 张新刚, 化学学报, 2024, 82, 105.)
doi: 10.6023/A23080395 |
|
|
(c)
doi: 10.1039/D3GC03471B |
|
|
(d)
doi: 10.1039/D3GC05041F |
|
| [12] |
(a)
doi: 10.1016/S0040-4039(01)89512-5 pmid: 24605155 |
|
(b)
doi: 10.3762/bjoc.10.32 pmid: 24605155 |
|
|
(c)
doi: 10.1021/acs.orglett.5b02439 pmid: 24605155 |
|
|
(d)
doi: 10.1039/c3cc44271c pmid: 24605155 |
|
| [13] |
(a)
doi: 10.1002/chem.v20.25 |
|
(b)
doi: 10.1016/j.tet.2022.132694 |
|
| [14] |
(a)
doi: 10.1016/S0040-4039(00)97899-7 |
|
(b)
doi: 10.1016/S0040-4039(00)87332-3 |
|
| [15] |
(a)
doi: 10.1021/ja409941r |
|
(b)
doi: 10.1021/jacs.5b09888 |
|
|
(c)
doi: 10.1002/anie.v55.1 |
|
| [16] |
doi: 10.1002/anie.v56.47 |
| [17] |
(a)
doi: 10.1021/acs.joc.6b01620 |
|
(b)
doi: 10.1021/acs.orglett.9b04504 |
|
|
(c)
|
|
| [18] |
doi: 10.1021/acs.orglett.4c02232 |
| [19] |
doi: 10.1039/D4OB01496K |
| [20] |
doi: 10.1039/D3QO01331F |
| [21] |
|
| [22] |
(a)
doi: 10.1039/D4QO00778F |
|
(b)
doi: 10.1021/acscatal.5c01133 |
|
|
(c)
doi: 10.6023/A25010008 |
|
|
(李国凯, 朱滨锋, 胡涛, 樊瑞峰, 孙蔚青, 和振秀, 陈景超, 樊保敏, 化学学报, 2025, 83, 319.)
|
|
| [23] |
doi: 10.1021/acs.orglett.3c01787 |
| [24] |
doi: 10.1021/acs.orglett.4c02316 |
| [25] |
doi: 10.1021/acscatal.5b02204 |
| [26] |
|
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||