Acta Chimica Sinica ›› 2025, Vol. 83 ›› Issue (12): 1465-1471.DOI: 10.6023/A25070265 Previous Articles Next Articles
Communication
朱子琦a,*,†( ), 叶乐华a,†, 吴怡a,†, 郭梓圻a, 石枫a,b,*(
), 叶乐华a,†, 吴怡a,†, 郭梓圻a, 石枫a,b,*( )
)
投稿日期:2025-07-30
发布日期:2025-10-10
基金资助:
Ziqi Zhua,*( ), Lehua Yea, Yi Wua, Ziqi Guoa, Feng Shia,b,*(
), Lehua Yea, Yi Wua, Ziqi Guoa, Feng Shia,b,*( )
)
Received:2025-07-30
Published:2025-10-10
Contact:
* E-mail: zzq@cczu.edu.cn;fshi@jsnu.edu.cn
About author:Supported by:Share
Ziqi Zhu, Lehua Ye, Yi Wu, Ziqi Guo, Feng Shi. o-Quinone Methide-Involved Chemoselective and Diastereoselective [2+3] Annulation[J]. Acta Chimica Sinica, 2025, 83(12): 1465-1471.
| Entry | Metal | Ligand | Solvent | Yield/% | dr |
|---|---|---|---|---|---|
| 1 | Pd2(dba)3 | PPh3 | MeCN | 84 | >95∶5 |
| 2 | Pd(PPh3)2Cl2 | PPh3 | MeCN | 12 | >95∶5 |
| 3 | [Ir(cod)Cl]2 | PPh3 | MeCN | N.R. | — |
| 4 | CuOTf | PPh3 | MeCN | N.R. | — |
| 5 | Pd2(dba)3 | dppp | MeCN | 40 | >95∶5 |
| 6 | Pd2(dba)3 | dppf | MeCN | 36 | >95∶5 |
| 7 | Pd2(dba)3 | PPh3 | DCE | 95 | >95∶5 |
| 8 | Pd2(dba)3 | PPh3 | EtOAc | 83 | >95∶5 |
| 9 | Pd2(dba)3 | PPh3 | DMF | 62 | >95∶5 |
| 10 | Pd2(dba)3 | PPh3 | Toluene | 82 | >95∶5 |
| 11 | Pd2(dba)3 | PPh3 | THF | 76 | >95∶5 |
| 12b | Pd2(dba)3 | PPh3 | DCE | 86 | >95∶5 |
| 13c | Pd2(dba)3 | PPh3 | DCE | 68 | >95∶5 |
| Entry | Metal | Ligand | Solvent | Yield/% | dr |
|---|---|---|---|---|---|
| 1 | Pd2(dba)3 | PPh3 | MeCN | 84 | >95∶5 |
| 2 | Pd(PPh3)2Cl2 | PPh3 | MeCN | 12 | >95∶5 |
| 3 | [Ir(cod)Cl]2 | PPh3 | MeCN | N.R. | — |
| 4 | CuOTf | PPh3 | MeCN | N.R. | — |
| 5 | Pd2(dba)3 | dppp | MeCN | 40 | >95∶5 |
| 6 | Pd2(dba)3 | dppf | MeCN | 36 | >95∶5 |
| 7 | Pd2(dba)3 | PPh3 | DCE | 95 | >95∶5 |
| 8 | Pd2(dba)3 | PPh3 | EtOAc | 83 | >95∶5 |
| 9 | Pd2(dba)3 | PPh3 | DMF | 62 | >95∶5 |
| 10 | Pd2(dba)3 | PPh3 | Toluene | 82 | >95∶5 |
| 11 | Pd2(dba)3 | PPh3 | THF | 76 | >95∶5 |
| 12b | Pd2(dba)3 | PPh3 | DCE | 86 | >95∶5 |
| 13c | Pd2(dba)3 | PPh3 | DCE | 68 | >95∶5 |
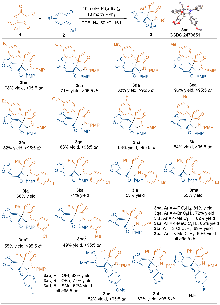

| Entry | Ligand | Solvent | Yield/% | dr | ee/% |
|---|---|---|---|---|---|
| 1 | L1 | MeCN | N.R. | — | — |
| 2 | L2 | MeCN | N.R. | — | — |
| 3 | L3 | MeCN | N.R. | — | — |
| 4 | L4 | MeCN | N.R. | — | — |
| 5 | L5 | MeCN | 80 | >95∶5 | 29 |
| 6 | L6 | MeCN | 23 | >95∶5 | -13 |
| 7 | L7 | MeCN | 67 | >95∶5 | 15 |
| 8 | L8 | MeCN | 88 | >95∶5 | -15 |
| 9 | L9 | MeCN | 62 | >95∶5 | -7 |
| 10 | L10 | MeCN | 72 | >95∶5 | 19 |
| 11 | L11 | MeCN | 41 | >95∶5 | 13 |
| 12 | L12 | MeCN | 25 | >95∶5 | 42 |
| 13 | L12 | DCE | 35 | >95∶5 | 9 |
| 14 | L12 | DMF | 14 | >95∶5 | 12 |
| 15 | L12 | THF | 49 | >95∶5 | 13 |
| 16 | L12 | Toluene | 58 | >95∶5 | 14 |
| Entry | Ligand | Solvent | Yield/% | dr | ee/% |
|---|---|---|---|---|---|
| 1 | L1 | MeCN | N.R. | — | — |
| 2 | L2 | MeCN | N.R. | — | — |
| 3 | L3 | MeCN | N.R. | — | — |
| 4 | L4 | MeCN | N.R. | — | — |
| 5 | L5 | MeCN | 80 | >95∶5 | 29 |
| 6 | L6 | MeCN | 23 | >95∶5 | -13 |
| 7 | L7 | MeCN | 67 | >95∶5 | 15 |
| 8 | L8 | MeCN | 88 | >95∶5 | -15 |
| 9 | L9 | MeCN | 62 | >95∶5 | -7 |
| 10 | L10 | MeCN | 72 | >95∶5 | 19 |
| 11 | L11 | MeCN | 41 | >95∶5 | 13 |
| 12 | L12 | MeCN | 25 | >95∶5 | 42 |
| 13 | L12 | DCE | 35 | >95∶5 | 9 |
| 14 | L12 | DMF | 14 | >95∶5 | 12 |
| 15 | L12 | THF | 49 | >95∶5 | 13 |
| 16 | L12 | Toluene | 58 | >95∶5 | 14 |
| [1] |
For reviews: (a)
doi: 10.1039/C1CS15156H pmid: 29560980 |
|
(b)
doi: 10.1002/adsc.v355.6 pmid: 29560980 |
|
|
(c)
doi: 10.1021/cs401172r pmid: 29560980 |
|
|
(d)
doi: 10.1002/adsc.v360.2 pmid: 29560980 |
|
|
(e)
doi: 10.1039/C6CS00825A pmid: 29560980 |
|
|
(f)
doi: 10.1039/C7OB02686B pmid: 29560980 |
|
|
(g)
doi: 10.1039/C8CC02364F pmid: 29560980 |
|
|
(h)
doi: 10.1021/acs.accounts.0c00697 pmid: 29560980 |
|
|
(i)
doi: 10.1021/acs.accounts.2c00764 pmid: 29560980 |
|
|
(j)
doi: 10.1021/acs.accounts.7b00377 pmid: 29560980 |
|
| [2] |
For recent examples: (a)
doi: 10.1016/j.chempr.2022.06.014 |
|
(b)
|
|
|
(c)
doi: 10.1039/D4QO01424C |
|
|
(d)
doi: 10.1021/acscatal.4c05266 |
|
|
(e)
|
|
|
(f)
|
|
|
(g)
doi: 10.6023/cjoc202501015 |
|
|
(陈刚, 陈东, 聂广杰, 李林轩, 姚辉, 王能中, 黄年玉, 有机化学, 2025, 45, 2139.)
doi: 10.6023/cjoc202501015 |
|
|
(h)
doi: 10.6023/cjoc202500004 |
|
|
(戈丁浩, 石枫, 有机化学, 2025, 45, 717.)
doi: 10.6023/cjoc202500004 |
|
|
(i)
doi: 10.6023/cjoc202406001 |
|
|
(j)
doi: 10.6023/A25050172 |
|
|
(张杰豪, 徐明金, 古满珍, 马浩文, 蔡倩, 化学学报, 2025, 83, 667.)
|
|
|
(k)
doi: 10.6023/A24060191 |
|
|
(王玉, 于聪, 夏艳芳, 林蓝, 陈强, 王新波, 化学学报 2024, 82, 914.)
doi: 10.6023/A24060191 |
|
| [3] |
(a)
pmid: 26163220 |
|
(b)
doi: 10.1002/hlca.v87:6 pmid: 26163220 |
|
|
(c)
doi: 10.1039/C4OB02459A pmid: 26163220 |
|
|
(d)
doi: 10.1016/j.ejmech.2015.06.050 pmid: 26163220 |
|
| [4] |
(a)
doi: 10.1021/ol034646i |
|
(b)
doi: 10.1021/jo035058l |
|
| [5] |
(a)
doi: 10.1002/chem.v24.15 |
|
(b)
doi: 10.1021/acs.joc.3c01315 |
|
| [6] |
For reviews: (a)
doi: 10.1021/ar010105m pmid: 21999240 |
|
(b)
doi: 10.1021/jo201789k pmid: 21999240 |
|
|
(c)
doi: 10.1002/chem.v18.30 pmid: 21999240 |
|
|
(d)
doi: 10.1039/C8CS00274F pmid: 21999240 |
|
|
(e)
doi: 10.1021/acs.accounts.2c00486 pmid: 21999240 |
|
| [7] |
For reviews: (a)
doi: 10.1021/ar500330x |
|
(b)
doi: 10.3390/molecules200711733 |
|
|
(c)
doi: 10.1038/s44160-022-00072-x |
|
|
(d)
|
|
|
(王海清, 杨爽, 张宇辰, 石枫, 有机化学, 2023, 43, 974.)
doi: 10.6023/cjoc202211022 |
|
|
(e)
|
|
|
(杨爽, 王宁宜, 杭青青, 张宇辰, 石枫, 化学学报, 2023, 81, 793.)
doi: 10.6023/A23040192 |
|
| [8] |
For early examples: (a)
doi: 10.1021/ol802029e pmid: 18850717 |
|
(b)
doi: 10.1002/adsc.v351:17 pmid: 18850717 |
|
| [9] |
For recent examples: (a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
doi: 10.1021/acscatal.3c02851 |
|
|
(e)
doi: 10.1039/D4QO00141A |
|
|
(f)
doi: 10.1039/D4QO00148F |
|
|
(g)
doi: 10.1021/acs.joc.4c01080 |
|
|
(h)
doi: 10.1021/acs.orglett.5c02145 |
|
| [10] |
(a)
doi: 10.1021/acs.orglett.2c01137 |
|
(b)
|
|
| [11] |
(a)
doi: 10.1021/acs.orglett.3c02242 |
|
(b)
doi: 10.1021/acs.orglett.3c01883 |
|
|
(c)
doi: 10.1021/acs.joc.3c00707 |
|
|
(d)
doi: 10.1016/j.cclet.2024.109688 |
|
|
(e)
doi: 10.1021/acs.orglett.4c01003 |
|
| [12] |
doi: 10.1039/D3SC00112A |
| [13] |
For reviews: (a)
doi: 10.1021/cr050011g |
|
(b)
doi: 10.1021/acs.accounts.0c00113 |
|
|
(c)
doi: 10.1002/cjoc.v38.12 |
|
|
(d)
doi: 10.1021/acscatal.1c00081 |
|
|
(e)
doi: 10.1002/cjoc.v39.12 |
|
|
(f)
|
|
|
(g)
doi: 10.1007/s11426-023-1683-9 |
|
|
(h)
doi: 10.1021/jacs.2c12995 |
|
|
(i)
doi: 10.1016/j.fmre.2022.07.008 |
|
|
(j)
doi: 10.1021/acs.joc.5c00104 |
|
| [14] |
For reviews: (a)
|
|
(b)
doi: 10.1039/D4QO00515E |
|
| [15] |
For recent examples: (a)
doi: 10.1016/j.fmre.2022.01.002 |
|
(b)
doi: 10.1002/cjoc.v41.1 |
|
|
(c)
doi: 10.1007/s11426-023-1927-3 |
|
|
(d)
doi: 10.6023/cjoc202405040 |
|
|
(e)
doi: 10.1007/s11426-024-2472-2 |
|
| [16] |
(a)
|
|
(游书力, 朱霞珍, 候雪龙, 戴立信, 化学学报, 2001, 59, 1667.)
|
|
|
(b)
doi: 10.1021/cr020027w |
|
|
(c)
doi: 10.1039/C5CS00144G |
|
|
(d)
doi: 10.6023/cjoc201704034 |
|
|
(邓颖颍, 杨文, 杨新, 杨定乔, 有机化学 2017, 37, 3039.)
doi: 10.6023/cjoc201704034 |
|
|
(e)
doi: 10.6023/A18060237 |
|
|
(张毛毛, 骆元元, 陆良秋, 肖文精, 化学学报 2018, 76, 838.)
doi: 10.6023/A18060237 |
|
| [17] |
|
| [18] |
(a)
doi: 10.1021/ja409669r |
|
(b)
doi: 10.1021/acs.accounts.9b00029 |
| [1] | Shanshan Liu, Weiwei Dong, Zhenzhen Li, Yaoyao Zhang, Chao Li, Linyu Jiao. Ionic Liquids Controlled Switchable Synthesis of Diverse Bio-Based N-Heterocycles via Tandem Dehydrogenative Cyclization [J]. Acta Chimica Sinica, 2025, 83(5): 479-487. |
| [2] | Yucheng Yin, Lijing Leng, Xiaolong Lin, Yan Yu, Tian Cai, Qunli Luo. One-Pot Synthesis of 1,4-Bridged Dihydroisoquinoline-3-ones from Isoquinolinium Salts and Cyclic 1,3-Diketones [J]. Acta Chimica Sinica, 2022, 80(12): 1569-1575. |
| [3] | Tian Yawei, Zhou Gang, Zhao Xiaoming, Dan Wenyan. Selective Fluorination of 2-Aminopyrazine Derivatives in Aqueous Phase [J]. Acta Chim. Sinica, 2018, 76(12): 962-966. |
| [4] | Yan Wen-Guang, Wang Pan, Wang Lijia, Sun Xiu-Li, Tang Yong. Copper Catalyzed[3+2] Annulation of Indoles with 1,1,2,2-Tetrasubstituted Donor-Acceptor Cyclopropanes [J]. Acta Chim. Sinica, 2017, 75(8): 783-787. |
| [5] | Yang Lijun, Ma Junan. New Advances on Nucleophilic Phosphine-Triggered Annulation Reactions of Allenoates [J]. Acta Chim. Sinica, 2016, 74(2): 130-148. |
| [6] | Zhou Rong, Xiao Wei, Yin Xiang, Zhan Gu, Chen Yingchun. Diastereo- and Enantioselective [4+2] Cycloadditions of Cyclic Enones with Cyclic 1-Azadienes [J]. Acta Chimica Sinica, 2014, 72(7): 862-866. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||