Acta Chimica Sinica ›› 2021, Vol. 79 ›› Issue (5): 658-662.DOI: 10.6023/A21010005 Previous Articles Next Articles
Article
投稿日期:2021-01-08
发布日期:2021-02-23
通讯作者:
张龙力, 刘欢
基金资助:
Da Yanga, Longli Zhanga,*( ), Huan Liua,*(
), Huan Liua,*( ), Chaohe Yangb
), Chaohe Yangb
Received:2021-01-08
Published:2021-02-23
Contact:
Longli Zhang, Huan Liu
About author:Supported by:Share
Da Yang, Longli Zhang, Huan Liu, Chaohe Yang. Co-catalysis over Bi-functional Ligand Based Ir-catalyst for Tandem Hydroformylation-acetalization Reaction[J]. Acta Chimica Sinica, 2021, 79(5): 658-662.
| Entry | CO/H2 | P/Ir | MeOH (mL) | NMP (mL) | Conv.b/% | Sel.b/% | Yieldb/% | L/Bc | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehyde | Acetal | Octane | Isomer | ||||||||
| 1 | 4∶1 | 1∶1 | 2 | 2 | 99 | 13 | 68 | 13 | 6 | 68 | 5.4 |
| 2 | 3∶1 | 1∶1 | 2 | 2 | 99 | 9 | 61 | 22 | 8 | 61 | 4.8 |
| 3 | 5∶1 | 1∶1 | 2 | 2 | 92 | 21 | 62 | 11 | 6 | 57 | 5.2 |
| 4 | 4∶1 | 2∶1 | 2 | 2 | 98 | 19 | 60 | 13 | 8 | 60 | 6.0 |
| 5 | 4∶1 | 1∶2 | 2 | 2 | 99 | 19 | 71 | 7 | 3 | 71 | 4.2 |
| 6 | 4∶1 | 1∶2 | 3 | 2 | 98 | 10 | 80 | 8 | 3 | 78 | 4.2 |
| 7 | 4∶1 | 1∶2 | 4 | 2 | 97 | 8 | 81 | 7 | 4 | 79 | 4.5 |
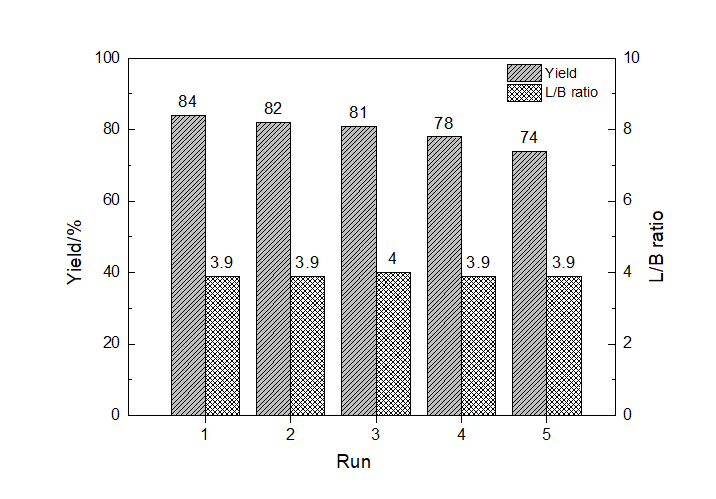
| 8 | 4∶1 | 1∶2 | 5 | 2 | 98 | 4 | 86 | 7 | 3 | 84 | 3.9 |
| 9 | 4∶1 | 1∶2 | 6 | 2 | 98 | 4 | 86 | 7 | 3 | 84 | 3.8 |
| 10 | 4∶1 | 1∶2 | 5 | — | 97 | 6 | 58 | 7 | 29 | 56 | 3.2 |
| 11d | 4∶1 | 1∶2 | 5 | 2 | 98 | 4 | 82 | 9 | 6 | 80 | 3.4 |
| 12e | 4∶1 | 1∶2 | 5 | 2 | 65 | 12 | 71 | 11 | 6 | 46 | 3.6 |
| Entry | CO/H2 | P/Ir | MeOH (mL) | NMP (mL) | Conv.b/% | Sel.b/% | Yieldb/% | L/Bc | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehyde | Acetal | Octane | Isomer | ||||||||
| 1 | 4∶1 | 1∶1 | 2 | 2 | 99 | 13 | 68 | 13 | 6 | 68 | 5.4 |
| 2 | 3∶1 | 1∶1 | 2 | 2 | 99 | 9 | 61 | 22 | 8 | 61 | 4.8 |
| 3 | 5∶1 | 1∶1 | 2 | 2 | 92 | 21 | 62 | 11 | 6 | 57 | 5.2 |
| 4 | 4∶1 | 2∶1 | 2 | 2 | 98 | 19 | 60 | 13 | 8 | 60 | 6.0 |
| 5 | 4∶1 | 1∶2 | 2 | 2 | 99 | 19 | 71 | 7 | 3 | 71 | 4.2 |
| 6 | 4∶1 | 1∶2 | 3 | 2 | 98 | 10 | 80 | 8 | 3 | 78 | 4.2 |
| 7 | 4∶1 | 1∶2 | 4 | 2 | 97 | 8 | 81 | 7 | 4 | 79 | 4.5 |
| 8 | 4∶1 | 1∶2 | 5 | 2 | 98 | 4 | 86 | 7 | 3 | 84 | 3.9 |
| 9 | 4∶1 | 1∶2 | 6 | 2 | 98 | 4 | 86 | 7 | 3 | 84 | 3.8 |
| 10 | 4∶1 | 1∶2 | 5 | — | 97 | 6 | 58 | 7 | 29 | 56 | 3.2 |
| 11d | 4∶1 | 1∶2 | 5 | 2 | 98 | 4 | 82 | 9 | 6 | 80 | 3.4 |
| 12e | 4∶1 | 1∶2 | 5 | 2 | 65 | 12 | 71 | 11 | 6 | 46 | 3.6 |
| Entry | Metal | Ligand | Conv.b/ % | Sel.b/% | Yieldb/ % | L/Bb | |
|---|---|---|---|---|---|---|---|
| Aldehyde | Acetal | ||||||
| 1 | [Ir(COD)Cl]2 | PPh3 | 36 | 46 | — | — | 2.7 |
| 2 | [Ir(COD)Cl]2 | L1 | 75 | 72 | — | — | 4.4 |
| 3 | [Ir(COD)Cl]2 | L1+L4 | 91 | 10 | 73 | 66 | 3.2 |
| 4 | [Ir(COD)Cl]2 | L2 | 98 | 4 | 86 | 84 | 3.9 |
| 5 | [Ir(COD)Cl]2 | L3 | 98 | 4 | 78 | 76 | 3.5 |
| 6 | [Rh(COD)Cl]2 | L2 | 89 | 8 | 86 | 77 | 1.1 |
| Entry | Metal | Ligand | Conv.b/ % | Sel.b/% | Yieldb/ % | L/Bb | |
|---|---|---|---|---|---|---|---|
| Aldehyde | Acetal | ||||||
| 1 | [Ir(COD)Cl]2 | PPh3 | 36 | 46 | — | — | 2.7 |
| 2 | [Ir(COD)Cl]2 | L1 | 75 | 72 | — | — | 4.4 |
| 3 | [Ir(COD)Cl]2 | L1+L4 | 91 | 10 | 73 | 66 | 3.2 |
| 4 | [Ir(COD)Cl]2 | L2 | 98 | 4 | 86 | 84 | 3.9 |
| 5 | [Ir(COD)Cl]2 | L3 | 98 | 4 | 78 | 76 | 3.5 |
| 6 | [Rh(COD)Cl]2 | L2 | 89 | 8 | 86 | 77 | 1.1 |
| Entry | Metal | Ligand | Conv.d/% | Yielde/% |
|---|---|---|---|---|
| 1b | — | L2 | 95 | 94 |
| 2b | [Ir(COD)Cl]2 | L2 | 93 | 91 |
| 3c | — | L2 | 94 | 92 |
| 4c | [Ir(COD)Cl]2 | L2 | 93 | 92 |
| 5c | — | — | 18 | 18 |
| Entry | Metal | Ligand | Conv.d/% | Yielde/% |
|---|---|---|---|---|
| 1b | — | L2 | 95 | 94 |
| 2b | [Ir(COD)Cl]2 | L2 | 93 | 91 |
| 3c | — | L2 | 94 | 92 |
| 4c | [Ir(COD)Cl]2 | L2 | 93 | 92 |
| 5c | — | — | 18 | 18 |
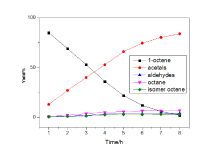

| Entry | Alkene | Alcohol | Major product | Yieldb/% | L/Bb |
|---|---|---|---|---|---|
| 1 | | MeOH | | 73 | 3.8 |
| 2 | | MeOH | | 82 | 3.7 |
| 3 | | MeOH | | 84 | 3.9 |
| 4 | | MeOH | | 86 | 2.1 |
| 5 | | MeOH | | 87 | 2.8 |
| 6 | | MeOH | | 81 | 0.2 |
| 7 | | MeOH | | 54 | — |
| 8c | | MeOH | | 80 | 0.45 |
| 9c | | MeOH | | 81 | 0.49 |
| 10c | | MeOH | | 81 | 0.47 |
| 11c | | MeOH | | 79 | 0.45 |
| 12c | | MeOH | | 76 | 0.25 |
| 13c | | MeOH | | 73 | 0.2 |
| 14 | | Glycol | | 75 | 3.0 |
| 15 | | isopropanol | | 45 | 3.2 |
| Entry | Alkene | Alcohol | Major product | Yieldb/% | L/Bb |
|---|---|---|---|---|---|
| 1 | | MeOH | | 73 | 3.8 |
| 2 | | MeOH | | 82 | 3.7 |
| 3 | | MeOH | | 84 | 3.9 |
| 4 | | MeOH | | 86 | 2.1 |
| 5 | | MeOH | | 87 | 2.8 |
| 6 | | MeOH | | 81 | 0.2 |
| 7 | | MeOH | | 54 | — |
| 8c | | MeOH | | 80 | 0.45 |
| 9c | | MeOH | | 81 | 0.49 |
| 10c | | MeOH | | 81 | 0.47 |
| 11c | | MeOH | | 79 | 0.45 |
| 12c | | MeOH | | 76 | 0.25 |
| 13c | | MeOH | | 73 | 0.2 |
| 14 | | Glycol | | 75 | 3.0 |
| 15 | | isopropanol | | 45 | 3.2 |
| [1] |
Liu, S.; Dai, X.; Wang, H.; Wang, X.; Shi, F. Chin. J. Chem. 2020, 38,139.
doi: 10.1002/cjoc.v38.2 |
| [2] |
Li, S.; Li, Z.; You, C.; Lv, H.; Zhang, X. Chin. J. Org. Chem. 2019, 39,1568. (in Chinese).
doi: 10.6023/cjoc201903044 |
|
( 李帅龙, 李庄星, 由才, 吕辉, 张绪穆, 有机化学, 2019, 39,1568.)
|
|
| [3] |
Kubis, C.; Selent, D.; Sawall, M.; Ludwig, R.; Neymeyr, K.; Baumann, W.; Franke, R.; Börner, A. Chem.-Eur. J. 2012, 18,8780.
doi: 10.1002/chem.v18.28 |
| [4] |
Franke, R.; Selent, D.; Börner, A. Chem. Rev. 2012, 112,5675.
doi: 10.1021/cr3001803 |
| [5] |
El A. i. B.; Tijani, J.; Fettouhi, M. Appl. Catal. Gen. 2006, 303,213.
doi: 10.1016/j.apcata.2006.02.004 |
| [6] |
Vieira, C. G.; da Silva, J. G.; Penna, C. A.A.; dos Santos, E. N.; Gusevskaya, E. V. Appl. Catal. Gen. 2010, 380,125.
doi: 10.1016/j.apcata.2010.03.045 |
| [7] |
Kalck, P.; Urrutigoïty, M. Chem. Rev. 2018, 118,3833.
doi: 10.1021/acs.chemrev.7b00667 |
| [8] |
Chen, C.; Jin, S.; Zhang, Z.; Wei, B.; Wang, H.; Zhang, K.; Lv, H.; Dong, X.-Q.; Zhang, X. J. Am. Chem. Soc. 2016, 138,9017.
doi: 10.1021/jacs.6b03596 |
| [9] |
Breit, B. Tetrahedron Lett. 1998, 39,5163.
doi: 10.1016/S0040-4039(98)01038-7 |
| [10] |
Wu, L.; Fleischer, I.; Jackstell, R.; Beller, M. J. Am. Chem. Soc. 2013, 135,3989.
doi: 10.1021/ja312271c |
| [11] |
Li, Y.-Q.; Zhou, Q.; Wang, D.-L.; Wang, P.; Lu, Y.; Liu, Y. Mol. Catal. 2017, 439,25.
|
| [12] |
Wang, P.; Wang, D.-L.; Liu, H.; Zhao, X.-L.; Lu, Y.; Liu, Y. Organometallics 2017, 36,2404.
doi: 10.1021/acs.organomet.7b00266 |
| [13] |
Fleischer, I.; Dyballa, K. M.; Jennerjahn, R.; Jackstell, R.; Franke, R.; Spannenberg, A.; Beller, M. Angew. Chem. Int. Ed. 2013, 52,2949.
doi: 10.1002/anie.201207133 |
| [14] |
Solsona, A.; Suades, J.; Mathieu, R. J. Organomet. Chem. 2003, 669,172.
doi: 10.1016/S0022-328X(03)00040-8 |
| [15] |
Wu, L.; Fleischer, I.; Jackstell, R.; Profir, I.; Franke, R.; Beller, M. J. Am. Chem. Soc. 2013, 135,14306.
doi: 10.1021/ja4060977 |
| [16] |
Jin, X.; Zhao, K.; Cui, F.; Kong, F.; Liu, Q. Green Chem. 2013, 15,3236.
doi: 10.1039/c3gc41231h |
| [17] |
Soulantica, K.; Sirol, S.; Koïnis, S.; Pneumatikakis, G.; Kalck, P. J. Organomet. Chem. 1995, 498,C10.
doi: 10.1016/0022-328X(95)05594-F |
| [18] |
Fernández, E.; Castillón, S. Tetrahedron Lett. 1994, 35,2361.
doi: 10.1016/0040-4039(94)85220-0 |
| [19] |
Norinder, J.; Rodrigues, C.; Börner, A. J. Mol. Catal. Chem. 2014, 391,139.
doi: 10.1016/j.molcata.2014.04.009 |
| [20] |
Wang, P.; Liu, H.; Li, Y.-Q.; Zhao, X.-L.; Lu, Y.; Liu, Y. Catal. Sci. Technol. 2016, 6,3854.
doi: 10.1039/C5CY01827G |
| [21] |
Li, Y.-Q.; Wang, P.; Liu, H.; Lu, Y.; Zhao, X.-L.; Liu, Y. Green Chem. 2016, 18,1798.
doi: 10.1039/C5GC02127H |
| [22] |
Wang, X.; Tian, S.-K. Tetrahedron Lett. 2007, 48,6010.
doi: 10.1016/j.tetlet.2007.06.132 |
| [23] |
Mukaiyama, T.; Matsui, S.; Kashiwagi, K. Chem. Lett. 1989,993.
|
| [24] |
Johnson, C. L.; Donkor, R. E.; Nawaz, W.; Karodia, N. Tetrahedron Lett. 2004, 45,7359.
doi: 10.1016/j.tetlet.2004.07.155 |
| [25] |
Piras, I.; Jennerjahn, R.; Jackstell, R.; Spannenberg, A.; Franke, R.; Beller, M. Angew. Chem. Int. Ed. 2011, 50,280.
doi: 10.1002/anie.v50.1 |
| [26] |
Kohl, G.; Rudolph, R.; Pritzkow, H.; Enders, M. Organometallics 2005, 24,4774.
doi: 10.1021/om050438d |
| [27] |
Wang, L.; Sowa, J. R.; Wang, C.; Lu, R. S.; Gassman, P. G.; Flood, T. C. Organometallics 1996, 15,4240.
doi: 10.1021/om9601099 |
| [28] |
Yang, D.; Liu, H.; Wang, D.-L.; Luo, Z.; Lu, Y.; Xia, F.; Liu, Y. Green Chem. 2018, 20,2588.
doi: 10.1039/C8GC00754C |
| [29] |
Yang, D.; Liu, H.; Liu, L.; Guo, W.-D.; Lu, Y.; Liu, Y. Green Chem. 2019, 21,5336.
doi: 10.1039/c9gc01887e |
| [30] |
Chi, X.; Cen, W.; Queenan, J. A.; Long, L.; Lynch, V. M.; Khashab, N. M.; Sessler, J. L. J. Am. Chem. Soc. 2019, 141,6468.
doi: 10.1021/jacs.9b01241 |
| [1] | Kongxi Qiu, Jie Li, Haowen Ma, Wei Zhou, Qian Cai. Recent Advances in the Construction of Nitrogen-Containing Heterocycles via Trapping Organocopper(I) Intermediates [J]. Acta Chimica Sinica, 2023, 81(1): 42-63. |
| [2] | He Ling, Gu Mengdi, Wang Dexian, Zhao Liang, Wang Meixiang. Tandem Heck Reaction of Tertiary Enamides: A Novel Access to trans-2,5-Disubstituted-3-Pyrroline Derivatives [J]. Acta Chim. Sinica, 2015, 73(10): 1018-1024. |
| [3] | Geng Haobing, Chen Shanshan, Sun Xi, Zhang Xiuli, Wang Lei. Copper-Catalyzed Tandem Etherification Reactions of 2-(2,2-Dibromovinyl)phenol Derivatives with Phenols [J]. Acta Chimica Sinica, 2014, 72(5): 595-601. |
| [4] | Su Ke, Jiang Hongbin, Zhu Deming, Fu Haiyan, Zheng Xueli, Yuan Maolin, Li Ruixiang, Chen Hua . Study on 1-Octene Hydroformylation Promoted by Cetyltrihydroxyethyl Ammonium Bromide in Aqueous/Organic Biphasic Solution [J]. Acta Chimica Sinica, 2013, 71(05): 844-848. |
| [5] | Wang Changhao, Li Yinghao, Jia Guoqing, Lu Shengmei, Liu Yan, Li Can. DNA Catalyzed Dithioacetalization in Water [J]. Acta Chimica Sinica, 2013, 71(01): 36-39. |
| [6] | LI Jian-Xiao, WANG Chao-Yang, XUE Fu-Ling, LUO Shi-He. Synthesis of Novel Tricyclic Fused 2(5H)-Furanone Derivatives with 1,2,3-Triazole Structure [J]. Acta Chimica Sinica, 2011, 69(23): 2835-2842. |
| [7] | TANG Dian-Yong. DFT Study on Mechanism of Acetylene Hydroformylation Catalyzed by Heterobinuclear Rh(I)-Cr Complex [J]. Acta Chimica Sinica, 2009, 67(12): 1303-1310. |
| [8] | LI Ming, LUO Xiao-Ling, TANG Dian-Yong. DFT Modeling Study of Ethylene Hydroformylation Catalyzed by Organic Phosphine-rhodium Catalyst [J]. Acta Chimica Sinica, 2004, 62(12): 1128-1133. |
| [9] | XIA CHUNGU;LI DAGANG;YANG WEIMAN;SUN YANWEN;YANG TIANXIANG. ^1H NMR study of rhodium phosphine complex under pressure [J]. Acta Chimica Sinica, 1990, 48(9): 890-893. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||