Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (2): 141-149.DOI: 10.6023/A21110526 Previous Articles Next Articles
Article
投稿日期:2021-11-20
发布日期:2021-12-23
通讯作者:
牛聪伟, 席真
基金资助:
Baifan Wang, Yinwu He, Xin Wen, Congwei Niu( ), Zhen Xi(
), Zhen Xi( )
)
Received:2021-11-20
Published:2021-12-23
Contact:
Congwei Niu, Zhen Xi
Supported by:Share
Baifan Wang, Yinwu He, Xin Wen, Congwei Niu, Zhen Xi. Prediction on the Resistance of Acetohydroxyacid Synthase Mutants to Herbicide Flumetsulam[J]. Acta Chimica Sinica, 2022, 80(2): 141-149.
| MB-QSAR/CoMFA | ||||||
|---|---|---|---|---|---|---|
| A-I | A-II | A-III | ||||
| A-II-3 | A-II-4 | A-II-5 | A-II-6 | |||
| ONCa | 5 | 5 | 5 | 5 | 5 | 5 |
| q2(LOO)b | 0.580 | 0.666 | 0.673 | 0.691 | 0.684 | 0.372 |
| SEEc | 0.315 | 0.261 | 0.256 | 0.259 | 0.264 | 0.401 |
| r2d | 0.922 | 0.947 | 0.948 | 0.947 | 0.945 | 0.874 |
| F-valuee | 125.318 | 187.64 | 195.036 | 190.608 | 182.745 | 73.208 |
| (5, 53) | (5, 53) | (5, 53) | (5, 53) | (5, 53) | (5, 53) | |
| r2predf | 0.725 | 0.746 | 0.747 | 0.759 | 0.754 | 0.621 |
| Contributionsg | ||||||
| S | 0.657 | 0.709 | 0.706 | 0.695 | 0.701 | 0.809 |
| E | 0.343 | 0.291 | 0.294 | 0.305 | 0.299 | 0.191 |
| MB-QSAR/CoMFA | ||||||
|---|---|---|---|---|---|---|
| A-I | A-II | A-III | ||||
| A-II-3 | A-II-4 | A-II-5 | A-II-6 | |||
| ONCa | 5 | 5 | 5 | 5 | 5 | 5 |
| q2(LOO)b | 0.580 | 0.666 | 0.673 | 0.691 | 0.684 | 0.372 |
| SEEc | 0.315 | 0.261 | 0.256 | 0.259 | 0.264 | 0.401 |
| r2d | 0.922 | 0.947 | 0.948 | 0.947 | 0.945 | 0.874 |
| F-valuee | 125.318 | 187.64 | 195.036 | 190.608 | 182.745 | 73.208 |
| (5, 53) | (5, 53) | (5, 53) | (5, 53) | (5, 53) | (5, 53) | |
| r2predf | 0.725 | 0.746 | 0.747 | 0.759 | 0.754 | 0.621 |
| Contributionsg | ||||||
| S | 0.657 | 0.709 | 0.706 | 0.695 | 0.701 | 0.809 |
| E | 0.343 | 0.291 | 0.294 | 0.305 | 0.299 | 0.191 |


| 力场组合 | SHA | SA | SH | SEA | SEHA | SEHDA | SDA | EHA | S |
|---|---|---|---|---|---|---|---|---|---|
| ONC | 6 | 6 | 5 | 6 | 6 | 6 | 5 | 6 | 4 |
| q2(LOO)a | 0.625 | 0.611 | 0.565 | 0.594 | 0.590 | 0.552 | 0.532 | 0.547 | 0.536 |
| SEE | 0.228 | 0.249 | 0.329 | 0.241 | 0.237 | 0.232 | 0.317 | 0.288 | 0.431 |
| r2 | 0.960 | 0.952 | 0.915 | 0.955 | 0.957 | 0.959 | 0.921 | 0.936 | 0.851 |
| F-value | 208.110 | 172.876 | 113.771 | 184.961 | 192.019 | 200.976 | 123.593 | 127.218 | 77.340 |
| r2pred | 0.619 | 0.536 | 0.468 | 0.608 | 0.581 | 0.417 | 0.361 | 0.556 | 0.708 |
| Contributionsb | |||||||||
| S | 0.270 | 0.340 | 0.571 | 0.204 | 0.176 | 0.118 | 0.177 | 1.000 | |
| E | 0.364 | 0.319 | 0.217 | 0.397 | |||||
| H | 0.178 | 0.429 | 0.119 | 0.084 | 0.159 | ||||
| D | 0.321 | 0.444 | |||||||
| A | 0.552 | 0.660 | 0.432 | 0.386 | 0.259 | 0.379 | 0.444 |
| 力场组合 | SHA | SA | SH | SEA | SEHA | SEHDA | SDA | EHA | S |
|---|---|---|---|---|---|---|---|---|---|
| ONC | 6 | 6 | 5 | 6 | 6 | 6 | 5 | 6 | 4 |
| q2(LOO)a | 0.625 | 0.611 | 0.565 | 0.594 | 0.590 | 0.552 | 0.532 | 0.547 | 0.536 |
| SEE | 0.228 | 0.249 | 0.329 | 0.241 | 0.237 | 0.232 | 0.317 | 0.288 | 0.431 |
| r2 | 0.960 | 0.952 | 0.915 | 0.955 | 0.957 | 0.959 | 0.921 | 0.936 | 0.851 |
| F-value | 208.110 | 172.876 | 113.771 | 184.961 | 192.019 | 200.976 | 123.593 | 127.218 | 77.340 |
| r2pred | 0.619 | 0.536 | 0.468 | 0.608 | 0.581 | 0.417 | 0.361 | 0.556 | 0.708 |
| Contributionsb | |||||||||
| S | 0.270 | 0.340 | 0.571 | 0.204 | 0.176 | 0.118 | 0.177 | 1.000 | |
| E | 0.364 | 0.319 | 0.217 | 0.397 | |||||
| H | 0.178 | 0.429 | 0.119 | 0.084 | 0.159 | ||||
| D | 0.321 | 0.444 | |||||||
| A | 0.552 | 0.660 | 0.432 | 0.386 | 0.259 | 0.379 | 0.444 |
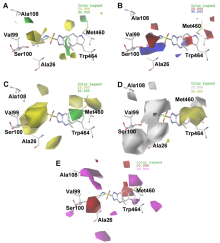

| 序列编号 | 种属 | Pred. pKi(氯磺隆) | Pred. pKi(氯嘧磺隆) | Pred. pKi(双草醚) | Pred. pKi(阔草清) |
|---|---|---|---|---|---|
| AAO53551.1 | 小麦 | 3.18 | 4.28 | 4.71 | 1.12 |
| ACD74789.1 | 籼稻 | 3.96 | 4.60 | 3.57 | 1.22 |
| NP_001151761.1 | 玉米 | 2.95 | 3.84 | 4.44 | 0.75 |
| CAA87083.1 | 棉花 | 3.51 | 6.03 | 4.86 | 0.25 |
| ADI56521.1 | 马铃薯 | 4.25 | 5.04 | 3.65 | 0.60 |
| P09342 | 烟草 | 3.70 | 4.43 | 4.64 | 1.14 |
| P09114 | 烟草 | 3.56 | 4.79 | 4.42 | 1.34 |
| ACU30048.1 | 大豆 | 3.38 | 4.50 | 4.90 | 0.63 |
| ACV84152.1 | 菜豆 | 2.48 | 4.18 | 3.77 | 1.40 |
| P27818 | 油菜 | 3.69 | 4.25 | 4.82 | 1.43 |
| P14874 | 油菜 | 3.35 | 4.11 | 4.43 | 1.61 |
| P27819 | 油菜 | 3.61 | 4.26 | 4.82 | 1.36 |
| XP_002511176.1 | 蓖麻 | 3.58 | 5.84 | 5.15 | 0.35 |
| EEF51778.1 | 蓖麻 | 3.58 | 5.84 | 5.15 | 0.35 |
| ACR55067.1 | 燕麦 | 3.74 | 5.39 | 4.89 | 1.52 |
| AAT07322.1 | 向日葵 | 3.51 | 5.17 | 4.75 | 1.24 |
| AAT07327.1 | 向日葵 | 3.89 | 5.10 | 4.74 | 0.67 |
| AAT07329.1 | 向日葵 | 3.72 | 3.91 | 4.27 | 2.30 |
| AEL89170.1 | 臭甘菊 | 4.75 | 5.11 | 4.82 | 1.28 |
| AEL89171.1 | 臭甘菊 | 4.48 | 4.89 | 5.15 | 1.06 |
| ABY57315.1 | 苜蓿 | 3.58 | 5.07 | 3.62 | 0.58 |
| ABY57318.1 | 苜蓿 | 3.70 | 5.02 | 3.34 | 0.51 |
| AAK50821.1 | 藜 | 4.14 | 4.63 | 5.27 | 0.97 |
| AAK50820.1 | 藜 | 4.12 | 4.76 | 5.31 | 1.04 |
| ABM92357.2 | 莎草 | 3.42 | 4.90 | 4.01 | 0.80 |
| ADQ54157.1 | 猪毛菜 | 4.15 | 5.24 | 4.84 | 0.90 |
| ACF47582.1 | 苣荬菜 | 3.58 | 4.65 | 4.83 | 1.33 |
| P17597 | 拟南芥 | 3.91 (7.04) | 4.66 (7.50) | 3.96 (6.09) | 0.37 (6.30) |
| P36620 | 粟酒裂殖酵母 | 4.22 (6.11) | 4.34 (8.15) | 5.51 (5.12) | 2.02 (3.95) |
| P00893 | 大肠杆菌I | 3.45 (3.32) | 2.90 (3.30) | 3.05 | 0.86 |
| P00892 | 大肠杆菌II | 6.29 (6.49) | 7.15 (7.66) | 4.98 (4.62) | 5.68 (5.73) |
| P08142 | 大肠杆菌III | 4.38 (3.81) | 4.02 (3.73) | 3.45 | 2.23 (3.22) |
| P37251 | 枯草芽孢杆菌 | 4.81 (4.78) | 4.25 (2.94) | 4.06 (2.87) | 2.33 (3.94) |
| P42463 | 谷氨酸棒杆菌 | 5.23 | 4.09 | 3.16 | 4.35 |
| P0A622 | 结核分支杆菌 | 3.53 | 3.80 | 3.74 | 3.99 |
| P0A623 | 牛分支杆菌 | 3.53 | 3.80 | 3.74 | 3.99 |
| P45261 | 流感嗜血杆菌 | 5.33 | 5.77 | 3.71 | 2.52 |
| O33112 | 麻风病分支杆菌 | 3.67 | 4.31 | 3.41 | 3.09 |
| Q59498 | 副结核杆菌亚种 | 4.54 | 4.16 | 3.86 | 3.50 |
| P57321 | 宿主豆长管蚜内共生菌 | 4.34 | 4.77 | 4.69 | 2.37 |
| O85293 | 宿主麦二叉蚜内共生菌 | 4.06 | 4.67 | 4.93 | 1.85 |
| Q89AP7 | 宿主木瘿蚜属内共生菌 | 4.01 | 4.12 | 4.39 | 2.50 |
| Q02137 | 乳酸乳球菌 | 5.27 | 4.49 | 2.79 | 3.55 |
| P40811 | 鼠伤寒沙门菌 | 4.38 | 4.23 | 3.75 | 2.47 |
| Q9RQ65 | 宿主角倍蚜内共生菌 | 3.64 | 4.08 | 4.66 | 2.59 |
| 序列编号 | 种属 | Pred. pKi(氯磺隆) | Pred. pKi(氯嘧磺隆) | Pred. pKi(双草醚) | Pred. pKi(阔草清) |
|---|---|---|---|---|---|
| AAO53551.1 | 小麦 | 3.18 | 4.28 | 4.71 | 1.12 |
| ACD74789.1 | 籼稻 | 3.96 | 4.60 | 3.57 | 1.22 |
| NP_001151761.1 | 玉米 | 2.95 | 3.84 | 4.44 | 0.75 |
| CAA87083.1 | 棉花 | 3.51 | 6.03 | 4.86 | 0.25 |
| ADI56521.1 | 马铃薯 | 4.25 | 5.04 | 3.65 | 0.60 |
| P09342 | 烟草 | 3.70 | 4.43 | 4.64 | 1.14 |
| P09114 | 烟草 | 3.56 | 4.79 | 4.42 | 1.34 |
| ACU30048.1 | 大豆 | 3.38 | 4.50 | 4.90 | 0.63 |
| ACV84152.1 | 菜豆 | 2.48 | 4.18 | 3.77 | 1.40 |
| P27818 | 油菜 | 3.69 | 4.25 | 4.82 | 1.43 |
| P14874 | 油菜 | 3.35 | 4.11 | 4.43 | 1.61 |
| P27819 | 油菜 | 3.61 | 4.26 | 4.82 | 1.36 |
| XP_002511176.1 | 蓖麻 | 3.58 | 5.84 | 5.15 | 0.35 |
| EEF51778.1 | 蓖麻 | 3.58 | 5.84 | 5.15 | 0.35 |
| ACR55067.1 | 燕麦 | 3.74 | 5.39 | 4.89 | 1.52 |
| AAT07322.1 | 向日葵 | 3.51 | 5.17 | 4.75 | 1.24 |
| AAT07327.1 | 向日葵 | 3.89 | 5.10 | 4.74 | 0.67 |
| AAT07329.1 | 向日葵 | 3.72 | 3.91 | 4.27 | 2.30 |
| AEL89170.1 | 臭甘菊 | 4.75 | 5.11 | 4.82 | 1.28 |
| AEL89171.1 | 臭甘菊 | 4.48 | 4.89 | 5.15 | 1.06 |
| ABY57315.1 | 苜蓿 | 3.58 | 5.07 | 3.62 | 0.58 |
| ABY57318.1 | 苜蓿 | 3.70 | 5.02 | 3.34 | 0.51 |
| AAK50821.1 | 藜 | 4.14 | 4.63 | 5.27 | 0.97 |
| AAK50820.1 | 藜 | 4.12 | 4.76 | 5.31 | 1.04 |
| ABM92357.2 | 莎草 | 3.42 | 4.90 | 4.01 | 0.80 |
| ADQ54157.1 | 猪毛菜 | 4.15 | 5.24 | 4.84 | 0.90 |
| ACF47582.1 | 苣荬菜 | 3.58 | 4.65 | 4.83 | 1.33 |
| P17597 | 拟南芥 | 3.91 (7.04) | 4.66 (7.50) | 3.96 (6.09) | 0.37 (6.30) |
| P36620 | 粟酒裂殖酵母 | 4.22 (6.11) | 4.34 (8.15) | 5.51 (5.12) | 2.02 (3.95) |
| P00893 | 大肠杆菌I | 3.45 (3.32) | 2.90 (3.30) | 3.05 | 0.86 |
| P00892 | 大肠杆菌II | 6.29 (6.49) | 7.15 (7.66) | 4.98 (4.62) | 5.68 (5.73) |
| P08142 | 大肠杆菌III | 4.38 (3.81) | 4.02 (3.73) | 3.45 | 2.23 (3.22) |
| P37251 | 枯草芽孢杆菌 | 4.81 (4.78) | 4.25 (2.94) | 4.06 (2.87) | 2.33 (3.94) |
| P42463 | 谷氨酸棒杆菌 | 5.23 | 4.09 | 3.16 | 4.35 |
| P0A622 | 结核分支杆菌 | 3.53 | 3.80 | 3.74 | 3.99 |
| P0A623 | 牛分支杆菌 | 3.53 | 3.80 | 3.74 | 3.99 |
| P45261 | 流感嗜血杆菌 | 5.33 | 5.77 | 3.71 | 2.52 |
| O33112 | 麻风病分支杆菌 | 3.67 | 4.31 | 3.41 | 3.09 |
| Q59498 | 副结核杆菌亚种 | 4.54 | 4.16 | 3.86 | 3.50 |
| P57321 | 宿主豆长管蚜内共生菌 | 4.34 | 4.77 | 4.69 | 2.37 |
| O85293 | 宿主麦二叉蚜内共生菌 | 4.06 | 4.67 | 4.93 | 1.85 |
| Q89AP7 | 宿主木瘿蚜属内共生菌 | 4.01 | 4.12 | 4.39 | 2.50 |
| Q02137 | 乳酸乳球菌 | 5.27 | 4.49 | 2.79 | 3.55 |
| P40811 | 鼠伤寒沙门菌 | 4.38 | 4.23 | 3.75 | 2.47 |
| Q9RQ65 | 宿主角倍蚜内共生菌 | 3.64 | 4.08 | 4.66 | 2.59 |
| [1] |
Hao, G. F.; Yang, G. F.; Zhan, C. G. J. Phys. Chem. B 2010, 114, 9663.
doi: 10.1021/jp102546s |
| [2] |
Chen, Y. Z.; Gu, X. L.; Cao, Z. W. J. Mol. Graph. Model. 2001, 19, 560.
doi: 10.1016/S1093-3263(01)00091-2 |
| [3] |
Cao, Z. W.; Han, L. Y.; Zheng, C. J.; Ji, Z. L.; Chen, X.; Lin, H. H.; Chen, Y. Z. Drug Discov. Today 2005, 10, 521.
doi: 10.1016/S1359-6446(05)03377-5 |
| [4] |
Hauser, K.; Negron, C.; Albanese, S. K.; Ray, S.; Steinbrecher, T.; Abel, R.; Chodera, J. D.; Wang, L. Commun. Biol. 2018, 1, 70.
doi: 10.1038/s42003-018-0075-x |
| [5] |
Goodford, P. J. J. Med. Chem. 1985, 28, 849.
doi: 10.1021/jm00145a002 |
| [6] |
Kastenholz, M. A.; Pastor, M.; Cruciani, G.; Haaksma, E. E.; Fox, T. J. Med. Chem. 2000, 43, 3033.
doi: 10.1021/jm000934y |
| [7] |
Wang, T.; Wade, R. C. J. Med. Chem. 2001, 44, 961.
doi: 10.1021/jm001070j |
| [8] |
Murcia, M.; Morreale, A.; Ortiz, A. R. J. Med. Chem. 2006, 49, 6241.
doi: 10.1021/jm060350h |
| [9] |
Deng, Z.; Chuaqui, C.; Singh, J. J. Med. Chem. 2004, 47, 337.
doi: 10.1021/jm030331x |
| [10] |
Cramer, R. D.; Patterson, D. E.; Bunce, J. D. J. Am. Chem. Soc. 1988, 110, 5959.
doi: 10.1021/ja00226a005 |
| [11] |
He, Y. W.; Niu, C. W.; Wen, X.; Xi, Z. Mol. Inform. 2013, 32, 139.
doi: 10.1002/minf.201200144 |
| [12] |
He, Y. W.; Niu, C. W.; Wen, X.; Xi, Z. Chin. J. Chem. 2013, 31, 1171.
doi: 10.1002/cjoc.v31.9 |
| [13] |
He, Y. W.; Niu, C. W.; Li, H.; Wen, X.; Xi, Z. Sci. China Chem. 2013, 56, 286.
doi: 10.1007/s11426-013-4841-9 |
| [14] |
Subramanian, M. V.; Gerwick, B. C. Plant Physiol. Biochem. 1989, 277.
|
| [15] |
Hawkes, T. Monograph-British Crop Protection Council 1989, 42, 131.
|
| [16] |
Shaner, D.; Anderson, P. C.; Stidham, M. A. Plant. Physiol. 1984, 76, 545.
doi: 10.1104/pp.76.2.545 |
| [17] |
Ray, T. B. Plant. Physiol. 1984, 75, 823.
|
| [18] |
McCourt, J. A.; Duggleby, R. G. Amino Acids 2006, 31, 173.
doi: 10.1007/s00726-005-0297-3 |
| [19] |
Rost, T. L. J. Plant. Growth. Regul. 1984, 3, 51.
doi: 10.1007/BF02041991 |
| [20] |
Tranel, P. J.; Wright, T. R. Weed Sci. 2002, 50, 700.
doi: 10.1614/0043-1745(2002)050[0700:RROWTA]2.0.CO;2 |
| [21] |
Bernasconi, P.; Woodworth, A. R.; Rosen, B. A.; Subramanian, M. V.; Siehl, D. L. J. Biol. Chem. 1995, 270, 17381.
doi: 10.1074/jbc.270.29.17381 |
| [22] |
Mourad, G.; Williams, D.; King, J. Planta 1995, 196, 64.
|
| [23] |
Sibony, M.; Michel, A.; Haas, H.; Rubin, B.; Hurle, K. Weed Res. 2001, 41, 509.
doi: 10.1046/j.1365-3180.2001.00254.x |
| [24] |
Hattori, J.; Brown, D.; Mourad, G.; Labbé, H.; Ouellet, T.; Sunohara, G.; Rutledge, R.; King, J.; Miki, B. Mol. Gen. Genet. 1995, 246, 419.
doi: 10.1007/BF00290445 |
| [25] |
Mourad, G.; King, J. Planta 1992, 188, 491.
|
| [26] |
Niu, C. W. Ph.D. Dissertation, Nankai University, Tianjin, 2005. (in Chinese)
|
|
( 牛聪伟, 博士论文, 南开大学, 天津, 2005.)
|
|
| [27] |
Xi, Z.; Niu, C. W.; Li, Q. X.; Ouyang, D.; Ban, S. R. Chin. J. Pestic. Sci. 2005, 7, 215. (in Chinese)
|
|
( 席真, 牛聪伟, 李庆霞, 欧阳砥, 班树荣, 农药学学报, 2005, 7, 215.)
|
|
| [28] |
McCourt, J. A.; Pang, S. S.; King-Scott, J.; Guddat, L. W.; Duggleby, R. G. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 569.
doi: 10.1073/pnas.0508701103 |
| [29] |
Xi, Z.; Niu, C. W.; Ban, S. R.; Li, Q. X.; Ouyang, D.; Huang, M. Z. Chin. J. Pestic. Sci. 2005, 7, 311. (in Chinese)
|
|
( 席真, 牛聪伟, 班树荣, 李庆霞, 欧阳砥, 黄明智, 农药学学报, 2005, 7, 311.)
|
|
| [30] |
He, Y. W. Ph.D. Dissertation, Nankai University, Tianjin, 2013. (in Chinese)
|
|
( 何寅武, 博士论文, 南开大学, 天津, 2013.)
|
|
| [31] |
Morris, G. M.; Goodsell, D. S.; Halliday, R. S.; Huey, R.; Hart, W. E.; Belew, R. K.; Olson, A. J. J. Comput. Chem. 1998, 19, 1639.
doi: 10.1002/(ISSN)1096-987X |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||