Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (3): 255-258.DOI: 10.6023/A22010002 Previous Articles Next Articles
Special Issue: 中国科学院青年创新促进会合辑
Communication
投稿日期:2022-01-01
发布日期:2022-02-11
通讯作者:
王丽佳, 唐勇
作者简介:基金资助:
Long Zhenga, Lijia Wanga,b,c( ), Yong Tanga(
), Yong Tanga( )
)
Received:2022-01-01
Published:2022-02-11
Contact:
Lijia Wang, Yong Tang
About author:Supported by:Share
Long Zheng, Lijia Wang, Yong Tang. Intramolecular Ring-opening of Indole-cyclopropanes※[J]. Acta Chimica Sinica, 2022, 80(3): 255-258.
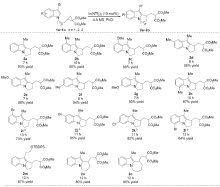
| Entry | Lewis acid | Solvent | Time/h | Conv./% | Yieldb/% |
|---|---|---|---|---|---|
| 1 | Cu(OTf)2 | DCM | 24 | 0 | — |
| 2 | Zn(OTf)2 | DCM | 24 | 0 | — |
| 3 | Fe(OTf)3 | DCM | 24 | 0 | — |
| 4 | La(OTf)3 | DCM | 24 | 0 | — |
| 5 | YbOTf)3 | DCM | 24 | 0 | — |
| 6 | Ga(OTf)3 | DCM | 24 | 15 | 15 |
| 7 | Sc(OTf)3 | DCM | 24 | 29 | 11 |
| 8 | In(OTf)3 | DCM | 24 | 76 | 66 |
| 9 | InCl3 | DCM | 24 | 8 | 7 |
| 10 | InBr3 | DCM | 24 | 38 | 34 |
| 11 | In(NTf2)3 | DCM | 24 | 86 | 82(80) c |
| 12 | In(NTf2)3 | THF | 72 | — | — |
| 13 | In(NTf2)3 | Toluene | 11 | 100 | 99(90) c |
| 14 | In(NTf2)3 | PhCl | 7 | 100 | 99(90) c |
| Entry | Lewis acid | Solvent | Time/h | Conv./% | Yieldb/% |
|---|---|---|---|---|---|
| 1 | Cu(OTf)2 | DCM | 24 | 0 | — |
| 2 | Zn(OTf)2 | DCM | 24 | 0 | — |
| 3 | Fe(OTf)3 | DCM | 24 | 0 | — |
| 4 | La(OTf)3 | DCM | 24 | 0 | — |
| 5 | YbOTf)3 | DCM | 24 | 0 | — |
| 6 | Ga(OTf)3 | DCM | 24 | 15 | 15 |
| 7 | Sc(OTf)3 | DCM | 24 | 29 | 11 |
| 8 | In(OTf)3 | DCM | 24 | 76 | 66 |
| 9 | InCl3 | DCM | 24 | 8 | 7 |
| 10 | InBr3 | DCM | 24 | 38 | 34 |
| 11 | In(NTf2)3 | DCM | 24 | 86 | 82(80) c |
| 12 | In(NTf2)3 | THF | 72 | — | — |
| 13 | In(NTf2)3 | Toluene | 11 | 100 | 99(90) c |
| 14 | In(NTf2)3 | PhCl | 7 | 100 | 99(90) c |
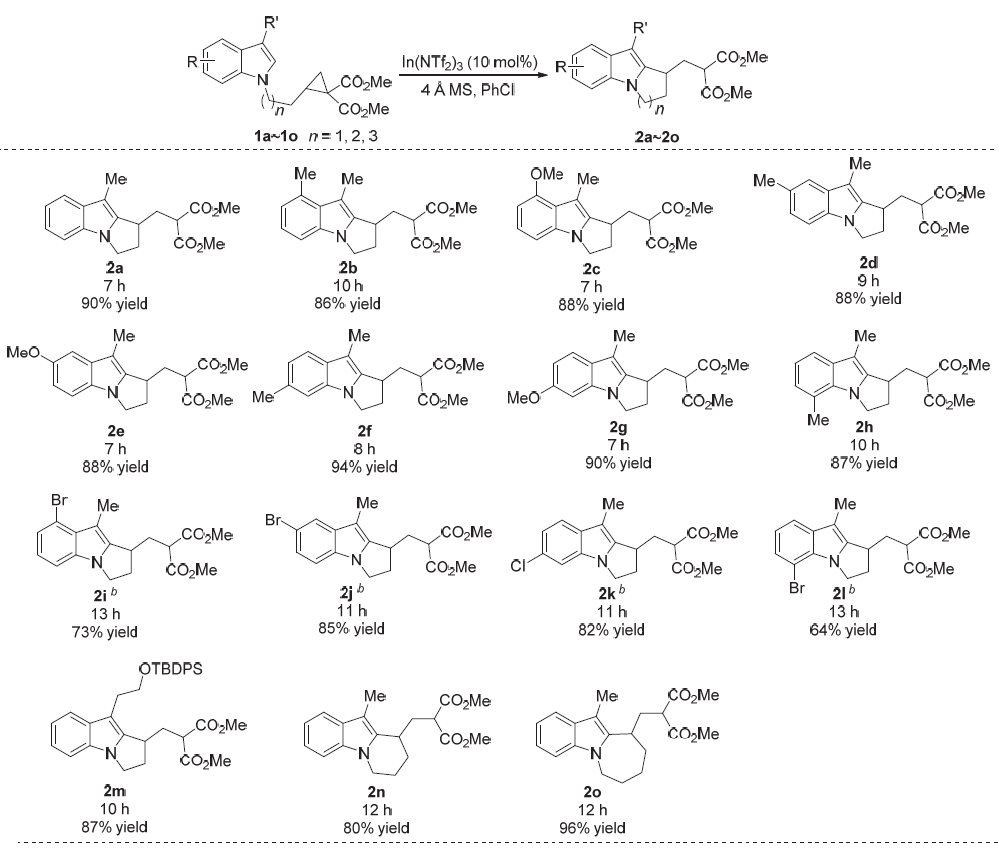
| [1] |
(a) Magnus, P.; Gallagher, T.; Brown, P.; Huffman, J. C. J. Am. Chem. Soc. 1984, 106, 2105.
doi: 10.1021/ja00319a034 |
|
(b) Gataullin, R. R. Russ. J. Org. Chem. 2009, 45, 321.
doi: 10.1134/S1070428009030014 |
|
|
(c) Dethe, D. H.; Erande, R. D.; Ranjan, A. J. Am. Chem. Soc. 2011, 133, 2864.
doi: 10.1021/ja1116974 |
|
|
(d) Vallakati, R.; May, J. A. J. Am. Chem. Soc. 2012, 134, 6936.
doi: 10.1021/ja301387k |
|
|
(e) Zeldin, R. M.; Toste, F. D. Chem. Sci. 2011, 2, 1706.
doi: 10.1039/c1sc00290b |
|
|
(f) Cheng, S.; Seo, J.; Huang, B. T.; Napolitano, T. Int. J. Oncol. 2016, 49, 1815.
doi: 10.3892/ijo.2016.3703 |
|
|
(g) Ghosh, A.; Bainbridge, D. T.; Stanley, L. M. J. Org. Chem. 2016, 81, 7945.
doi: 10.1021/acs.joc.6b01730 |
|
|
(h) Zhan, L.; Hu, W.; Wang, M.; Huang, B.; Long, Y.-Q. Acta Chim. Sinica 2021, 79, 903. (in Chinese)
doi: 10.6023/A21060252 |
|
|
(占林俊, 胡玮, 王梅, 黄斌, 龙亚秋, 化学学报, 2021, 79, 903).
doi: 10.6023/A21060252 |
|
|
(i) Zhou, B.; Liang, R.; Cao, Z.; Zhou, P.; Jia, Y. Acta Chim. Sinica 2021, 79, 176. (in Chinese)
doi: 10.6023/A20110520 |
|
|
(周波, 梁仁校, 曹中艳, 周平海, 贾义霞, 化学学报, 2021, 79, 176).
doi: 10.6023/A20110520 |
|
| [2] |
Hong, L.; Sun, W. S.; Liu, C. X.; Wang, L.; Wang, R. Chem. Eur. J. 2010, 16, 440.
doi: 10.1002/chem.v16:2 |
| [3] |
Patil, D. V.; Cavitt, M. A.; France, S. Org. Lett. 2011, 13, 5820.
doi: 10.1021/ol202431x |
| [4] |
Tejeda, J. E. C.; Landschoot, B. K.; Kerr, M. A. Org. Lett. 2016, 18, 2142.
doi: 10.1021/acs.orglett.6b00768 |
| [5] |
(a) Yip, K. T.; Yang, D. Org. Lett. 2011, 13, 2134.
doi: 10.1021/ol2006083 |
|
(b) Zhang, W.; Chen, P. H.; Liu, G. S. Angew. Chem. Int. Ed. 2017, 56, 5336.
doi: 10.1002/anie.201700889 |
|
| [6] |
For reviews and recent examples, see: (a) Wong, H. N. C.; Hon, M. Y.; Tse, C. W.; Yip, Y. C.; Tanko, J.; Hudlicky, T. Chem. Rev. 1989, 89, 165.
doi: 10.1021/cr00091a005 |
|
(b) Reissig, H. U.; Zimmer, R. Chem. Rev. 2003, 103, 1151.
doi: 10.1021/cr010016n |
|
|
(c) Carson, C. A.; Kerr, M. A. Chem. Soc. Rev. 2009, 38, 3051.
doi: 10.1039/b901245c |
|
|
(d) Tang, P.; Qin, Y. Synthesis 2012, 44, 2969.
doi: 10.1055/s-00000084 |
|
|
(e) Wang, Z. W. Synlett 2012, 23, 2311.
doi: 10.1055/s-00000083 |
|
|
(f) Reissig, H. U.; Zimmer, R. Angew. Chem. Int. Ed. 2015, 54, 5009.
doi: 10.1002/anie.v54.17 |
|
|
(g) Wang, Y.; Yu, Z.-X. Acc. Chem. Res. 2015, 48, 2288.
doi: 10.1021/acs.accounts.5b00037 |
|
|
(h) Qiu, Y.; Lu, K.; Wei, B.; Qian, Z.; He, Z. Chin. J. Org. Chem. 2021, 41, 4066. (in Chinese)
doi: 10.6023/cjoc202104036 |
|
|
(仇裕鹤, 鲁康辉, 韦邦尺, 潜振凯, 贺峥杰, 有机化学, 2021, 41, 4066.)
|
|
| [7] |
For reviews, see: (a) Yu, M.; Pagenkopf, B. L. Tetrahedron 2005, 61, 321.
doi: 10.1016/j.tet.2004.10.077 pmid: 25425071 |
|
(b) Rubin, M.; Rubina, M.; Gevorgyan, V. Chem. Rev. 2007, 107, 3117.
doi: 10.1021/cr050988l pmid: 25425071 |
|
|
(c) Cavitt, M. A.; Phun, L. H.; France, S. Chem. Soc. Rev. 2014, 43, 804.
doi: 10.1039/C3CS60238A pmid: 25425071 |
|
|
(d) de Nanteuil, F.; De Simone, F.; Frei, R.; Benfatti, F.; Serrano, E.; Waser, J. Chem. Commun. 2014, 50, 10912.
doi: 10.1039/C4CC03194F pmid: 25425071 |
|
|
(e) Schneider, T. F.; Kaschel, J.; Werz, D. B. Angew. Chem., Int. Ed. 2014, 53, 5504.
doi: 10.1002/anie.v53.22 pmid: 25425071 |
|
|
(f) Grover, H. K.; Emmett, M. R.; Kerr, M. A. Org. Biomol. Chem. 2015, 13, 655.
doi: 10.1039/c4ob02117g pmid: 25425071 |
|
| [8] |
(a) Beal, R. B.; Dombroski, M. A.; Snider, B. B. J. Org. Chem. 1986, 51, 4391.
doi: 10.1021/jo00373a010 |
|
(b) Tanimori, S.; Niki, T.; He, M. Q.; Nakayama, M. Heterocycles 1994, 38, 1533.
doi: 10.3987/COM-94-6714 |
|
|
(c) Kotsuki, H.; Arimura, K.; Maruzawa, R.; Ohshima, R. Synlett 1999, 5, 650.
|
|
| [9] |
(a) Xing, S. Y.; Pan, W. Y.; Liu, C.; Ren, J.; Wang, Z. W. Angew. Chem., Int. Ed. 2010, 49, 3215.
doi: 10.1002/anie.v49:18 |
|
(b) Zhu, W. J.; Fang, J.; Liu, Y.; Ren, J.; Wang, Z. W. Angew. Chem. Int. Ed. 2013, 52, 2032.
doi: 10.1002/anie.v52.7 |
|
| [10] |
For reviews, see: (a) Liao, S.; Sun, X. L.; Tang, Y. Acc. Chem. Res. 2014, 47, 2260.
doi: 10.1021/ar800104y |
|
(b) Wang, L.; Tang, Y. Isr. J. Chem. 2016, 56, 463.
doi: 10.1002/ijch.201500094 |
|
|
(c) Wang, L.; Zhou, J.; Tang, Y. Chin. J. Chem. 2018, 36, 1123.
doi: 10.1002/cjoc.v36.12 |
|
| [11] |
For recent examples, see: (a) Liu, H.-K.; Wang, S. R.; Song, X.-Y.; Zhao, L.-P.; Wang, L.; Tang, Y. Angew. Chem., Int. Ed. 2019, 58, 4345.
doi: 10.1002/anie.v58.13 |
|
(b) Zheng, Z.-B.; Cheng, W.-F.; Wang, L.; Zhu, J.; Sun, X.-L.; Tang, Y. Chin. J. Chem. 2020, 38, 1629.
doi: 10.1002/cjoc.v38.12 |
|
| [12] |
For selected examples of indoles with cyclopropanes, see: (a) Harrington, P.; Kerr, M. A. Tetrahedron Lett. 1997, 38, 5949.
doi: 10.1016/S0040-4039(97)01351-8 pmid: 23651295 |
|
(b) Kerr, M. A.; Keddy, R. G. Tetrahedron Lett. 1999, 40, 5671.
doi: 10.1016/S0040-4039(99)01107-7 pmid: 23651295 |
|
|
(c) England, D. B.; Kuss, T. D. O.; Keddy, R. G.; Kerr, M. A. J. Org. Chem. 2001, 66, 4704.
pmid: 23651295 |
|
|
(d) Bajtos, B.; Yu, M.; Zhao, H.; Pagenkopf, B. L. J. Am. Chem. Soc. 2007, 129, 9631.
doi: 10.1021/ja067821+ pmid: 23651295 |
|
|
(e) Wales, S. M.; Walker, M. M.; Johnson, J. S. Org. Lett. 2013, 15, 2558.
doi: 10.1021/ol4010646 pmid: 23651295 |
|
|
(f) Xiong, H.; Xu, H.; Liao, S.; Xie, Z.; Tang, Y. J. Am. Chem. Soc. 2013, 135, 7851.
doi: 10.1021/ja4042127 pmid: 23651295 |
|
|
(g) Liu, Q. J.; Yan, W. G.; Wang, L.; Zhang, X. P.; Tang, Y. Org. Lett. 2015, 17, 4014.
doi: 10.1021/acs.orglett.5b01909 pmid: 23651295 |
|
|
(h) Yan, W.-G.; Wang, P.; Wang, L.; Sun, X.-L.; Tang, Y. Acta Chim. Sinica 2017, 75, 783. (in Chinese)
doi: 10.6023/A17040146 pmid: 23651295 |
|
|
(严文广, 王盼, 王丽佳, 孙秀丽, 唐勇, 化学学报, 2017, 75, 783.)
doi: 10.6023/A17040146 pmid: 23651295 |
|
| [13] |
(a) Zheng, C.; Wu, Q.-F.; You, S.-L. J. Org. Chem. 2013, 78, 4357.
doi: 10.1021/jo400365e pmid: 23578142 |
|
(b) Zheng, C.; Xia, Z.-L.; You, S.-L. Chem 2018, 4, 1952.
doi: 10.1016/j.chempr.2018.06.006 pmid: 23578142 |
|
| [14] |
Zhu, J.; Liang, Y.; Wang, L.; Zheng, Z. B.; Houk, K. N.; Tang, Y. J. Am. Chem. Soc. 2014, 136, 6900.
doi: 10.1021/ja503117q |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||