Acta Chimica Sinica ›› 2023, Vol. 81 ›› Issue (10): 1341-1349.DOI: 10.6023/A23060292 Previous Articles Next Articles
Special Issue: 庆祝《化学学报》创刊90周年合辑
Original article
投稿日期:2023-06-16
发布日期:2023-08-15
作者简介:基金资助:
Yang Wang, Junjun Xiang, Congwu Ge, Xike Gao( )
)
Received:2023-06-16
Published:2023-08-15
Contact:
*E-mail: About author:Supported by:Share
Yang Wang, Junjun Xiang, Congwu Ge, Xike Gao. Study on Main Chain Structure Regulation and Properties of Conjugated Copolymers Based on 2,6-Azulene and 3,4-Propylenedioxythiophene★[J]. Acta Chimica Sinica, 2023, 81(10): 1341-1349.

| Polymer | Mn a/kDa | PDIa | Td b/℃ |
|---|---|---|---|
| P(AzProDOT-1) | 11.1 | 2.29 | 370 |
| P(AzProDOT-2) | 11.4 | 2.25 | 374 |
| P(AzProDOT-3) | 9.3 | 1.46 | 394 |
| Polymer | Mn a/kDa | PDIa | Td b/℃ |
|---|---|---|---|
| P(AzProDOT-1) | 11.1 | 2.29 | 370 |
| P(AzProDOT-2) | 11.4 | 2.25 | 374 |
| P(AzProDOT-3) | 9.3 | 1.46 | 394 |
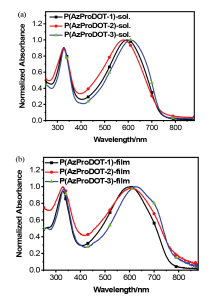
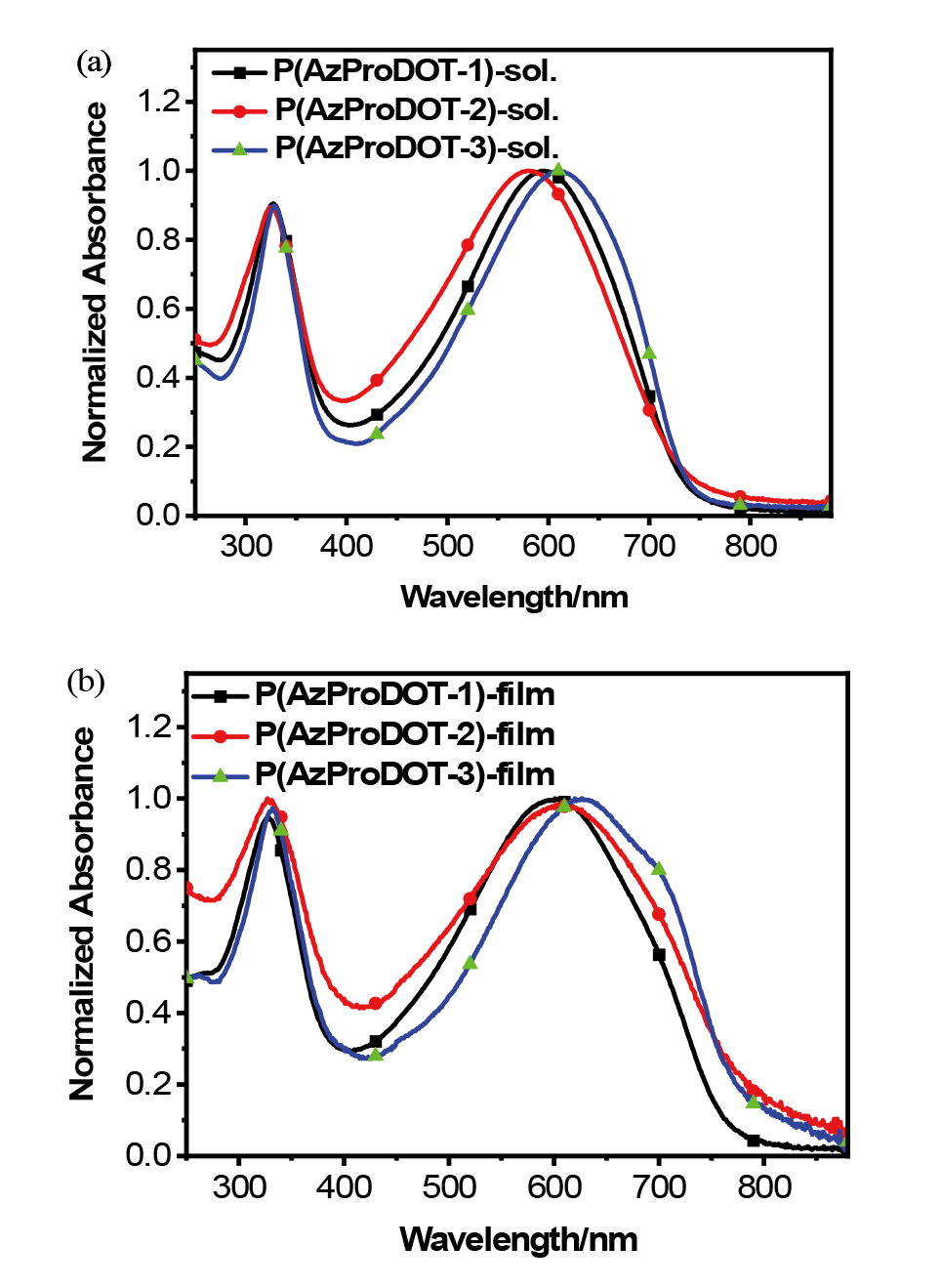
| Polymer | λmax /nm | Eopt ga/eV | ELUMO b/eV | EHOMO b/eV | ECV gc/eV | |
|---|---|---|---|---|---|---|
| Sol. | Film | |||||
| P(AzProDOT-1) | 594 | 601 | 1.60 | -3.50 | -5.36 | 1.86 |
| P(AzProDOT-2) | 587 | 603 | 1.55 | -3.48 | -5.38 | 1.90 |
| P(AzProDOT-3) | 613 | 626 | 1.58 | -3.48 | -5.40 | 1.92 |
| Polymer | λmax /nm | Eopt ga/eV | ELUMO b/eV | EHOMO b/eV | ECV gc/eV | |
|---|---|---|---|---|---|---|
| Sol. | Film | |||||
| P(AzProDOT-1) | 594 | 601 | 1.60 | -3.50 | -5.36 | 1.86 |
| P(AzProDOT-2) | 587 | 603 | 1.55 | -3.48 | -5.38 | 1.90 |
| P(AzProDOT-3) | 613 | 626 | 1.58 | -3.48 | -5.40 | 1.92 |
| [1] |
Lemal, D. M.; Goldman, G. D. J. Chem. Educ. 1988, 65, 923.
doi: 10.1021/ed065p923 |
| [2] |
(a) Koch, M.; Blacque, O.; Venkatesan, K. Org. Lett. 2012, 14, 1580.
doi: 10.1021/ol300327b |
|
(b) Tang, T.; Lin, T.; Wang, F.; He, C. J. Phys. Chem. B 2015, 119, 8176.
doi: 10.1021/acs.jpcb.5b01613 |
|
|
(c) Wang, F.; Lin, T. T.; He, C.; Chi, H.; Tang, T.; Lai, Y.-H. J. Mater. Chem. 2012, 22, 10448.
doi: 10.1039/c2jm31098h |
|
|
(d) Wang, F.; Lai, Y. H.; Kocherginsky, N. M.; Kosteski, Y. Y. Org. Lett. 2003, 5, 995.
doi: 10.1021/ol0274615 |
|
| [3] |
(a) Anderson, A. G.; Steckler, B. M. J. Am. Chem. Soc. 1959, 81, 4941.
doi: 10.1021/ja01527a046 |
|
(b) Michl, J.; Thulstrup, E. W. Tetrahedron 1976, 32, 205.
doi: 10.1016/0040-4020(76)87002-0 |
|
| [4] |
(a) Xin, H.; Li, J.; Lu, R. Q.; Gao, X.; Swager, T. M. J. Am. Chem. Soc. 2020, 142, 13598.
doi: 10.1021/jacs.0c06299 |
|
(b) Ran, H.; Duan, X.; Zheng, R.; Xie, F.; Chen, L.; Zhao, Z.; Han, R.; Lei, Z.; Hu, J. Y. ACS Appl. Mater. Interfaces 2020, 12, 23225.
doi: 10.1021/acsami.0c04552 |
|
|
(c) Yamaguchi, Y.; Maruya, Y.; Katagiri, H.; Nakayama, K.; Ohba, Y. Org. Lett. 2012, 14, 2316.
doi: 10.1021/ol3007327 |
|
|
(d) Hou, B.; Li, J.; Xin, H.; Yang, X.; Gao, H.; Peng, P.; Gao, X. Acta Chim. Sinica 2020, 78, 788 (in Chinese).
doi: 10.6023/A20050161 |
|
|
(侯斌, 李晶, 辛涵申, 杨笑迪, 高洪磊, 彭培珍, 高希珂, 化学学报, 2020, 78, 788)
|
|
|
(e) Peng, P.; Li, J.; Hou, B.; Xin, H.; Cheng, T.; Gao, X. Chin. J. Org. Chem. 2020, 40, 3916 (in Chinese).
doi: 10.6023/cjoc202005014 |
|
|
(彭培珍, 李晶, 侯斌, 辛涵申, 程探宇, 高希珂, 有机化学, 2020, 40, 3916)
|
|
| [5] |
(a) Yao, J.; Cai, Z.; Liu, Z.; Yu, C.; Luo, H.; Yang, Y.; Yang, S.; Zhang, G.; Zhang, D. Macromolecules 2015, 48, 2039.
doi: 10.1021/acs.macromol.5b00158 |
|
(b) Xin, H.; Li, J.; Ge, C.; Yang, X.; Xue, T.; Gao, X. Mater. Chem. Front. 2018, 2, 975.
doi: 10.1039/C8QM00047F |
|
|
(c) Chen, Y.; Zhu, Y.; Yang, D.; Zhao, S.; Zhang, L.; Yang, L.; Wu, J.; Huang, Y.; Xu, Z.; Lu, Z. Chemistry 2016, 22, 14527.
|
|
| [6] |
(a) Tzoganakis, N.; Feng, B.; Loizos, M.; Krassas, M.; Tsikritzis, D.; Zhuang, X.; Kymakis, E. J. Mater. Chem. C 2021, 9, 14709.
doi: 10.1039/D1TC02726C |
|
(b) Su, Y.; Li, H.; Miao, Y.; Tian, Y.; Cheng, M. Asian. J. Org. Chem. 2022, 11, e202200441.
|
|
|
(c) Zhu, W.; Zhou, K.; Fo, Y.; Li, Y.; Guo, B.; Zhang, X.; Zhou, X. Phys. Chem. Chem. Phys. 2022, 24, 18793.
doi: 10.1039/D2CP02036J |
|
| [7] |
(a) Umeyama, T.; Watanabe, Y.; Miyata, T.; Imahori, H. Chem. Lett. 2015, 44, 47.
doi: 10.1246/cl.140904 |
|
(b) Wang, X.; Ng, J. K.-P.; Jia, P.; Lin, T.; Cho, C. M.; Xu, J.; Lu, X.; He, C. Macromolecules 2009, 42, 5534.
doi: 10.1021/ma900847r |
|
|
(c) Wang, F.; Lai, Y.-H.; Han, M.-Y. Macromolecules 2004, 37, 3222.
doi: 10.1021/ma035335q |
|
|
(d) Wang, F.; Lai, Y.-H. Macromolecules 2003, 36, 536.
doi: 10.1021/ma025662i |
|
| [8] |
Murai, M.; Amir, E.; Amir, R. J.; Hawker, C. J. Chem. Sci. 2012, 3, 2721.
doi: 10.1039/c2sc20615c |
| [9] |
Xin, H.; Hou, B.; Gao, X. Acc. Chem. Res. 2021, 54, 1737.
doi: 10.1021/acs.accounts.0c00893 |
| [10] |
(a) Yamaguchi, Y.; Ogawa, K.; Nakayama, K.; Ohba, Y.; Katagiri, H. J. Am. Chem. Soc. 2013, 135, 19095.
doi: 10.1021/ja410696j |
|
(b) Yamaguchi, Y.; Takubo, M.; Ogawa, K.; Nakayama, K.; Koganezawa, T.; Katagiri, H. J. Am. Chem. Soc. 2016, 138, 11335.
doi: 10.1021/jacs.6b06877 |
|
| [11] |
Xiang, J.; Tan, W. L.; Zhang, J.; Wang, Y.; Duan, C.; McNeill, C. R.; Yang, X.; Ge, C.; Gao, X. Macromolecules 2022, 55, 8074.
doi: 10.1021/acs.macromol.2c01101 |
| [12] |
(a) Gao, H.; Ge, C.; Hou, B.; Xin, H.; Gao, X. ACS Macro Lett. 2019, 8, 1360.
doi: 10.1021/acsmacrolett.9b00657 |
|
(b) Hou, B.; Zhou, Z.; Yu, C.; Xue, X. S.; Zhang, J.; Yang, X.; Li, J.; Ge, C.; Wang, J.; Gao, X. ACS Macro Lett. 2022, 11, 680.
doi: 10.1021/acsmacrolett.2c00164 |
|
| [13] |
(a) Homyak, P.; Liu, Y.; Liu, F.; Russel, T. P.; Coughlin, E. B. Macromolecules 2015, 48, 6978.
doi: 10.1021/acs.macromol.5b01275 |
|
(b) Gobalasingham, N. S.; Pankow, R. M.; Ekiz, S.; Thompson, B. C. J. Mater. Chem. A 2017, 5, 14101.
doi: 10.1039/C7TA03980H |
|
|
(c) Broll, S.; Nübling, F.; Luzio, A.; Lentzas, D.; Komber, H.; Caironi, M.; Sommer, M. Macromolecules 2015, 48, 7481.
doi: 10.1021/acs.macromol.5b01843 |
|
|
(d) Akkuratov, A. V.; Prudnov, F. A.; Chernyak, A. V.; Kuznetsov, P. M.; Peregudov, A. S.; Troshin, P. A. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 776.
doi: 10.1002/pola.v57.7 |
|
| [14] |
(a) DiTullio, B. T.; Savagian, L. R.; Bardagot, O.; De Keersmaecker, M.; Osterholm, A. M.; Banerji, N.; Reynolds, J. R. J. Am. Chem. Soc. 2023, 145, 122.
doi: 10.1021/jacs.2c08850 |
|
(b) Mi, S.; Wu, J.; Liu, J.; Xu, Z.; Wu, X.; Luo, G.; Zheng, J.; Xu, C. ACS Appl. Mater. Interfaces 2015, 7, 27511.
doi: 10.1021/acsami.5b09717 |
|
|
(c) Luo, X.; Shen, H.; Perera, K.; Tran, D. T.; Boudouris, B. W.; Mei, J. ACS Macro Lett. 2021, 10, 1061.
doi: 10.1021/acsmacrolett.1c00328 |
|
|
(d) Yang, W.; Yue, H.-G.; Zhao, D.; Yan, H.; Cao, K.-L.; Zhao, J.-S.; Zhang, Q. Chinese J. Polym. Sci. 2021, 39, 147.
doi: 10.1007/s10118-021-2503-5 |
|
| [15] |
Meager, I.; Ashraf, R. S.; Rossbauer, S.; Bronstein, H.; Donaghey, J. E.; Marshall, J.; Schroeder, B. C.; Heeney, M.; Anthopoulos, T. D.; McCulloch, I. Macromolecules 2013, 46, 5961.
doi: 10.1021/ma401128s |
| [16] |
Fan, Q.; Martin-Jimenez, D.; Ebeling, D.; Krug, C. K.; Brechmann, L.; Kohlmeyer, C.; Hilt, G.; Hieringer, W.; Schirmeisen, A.; Gottfried, J. M. J. Am. Chem. Soc. 2019, 141, 17713.
doi: 10.1021/jacs.9b08060 |
| [17] |
Hou, B.; Li, J.; Zhou, Z. F.; Tan, W. L.; Yang, X. D.; Zhang, J. W.; McNeill, C. R.; Ge, C. W.; Wang, J. T.; Gao, X. K. ACS Mater. Lett. 2022, 4, 392.
|
| [18] |
Wang, Y.; Tan, W. L.; Xiang, J.; Ge, C.; McNeill, C. R.; Gao, X. ACS Macro Lett. 2023, 12, 487.
doi: 10.1021/acsmacrolett.3c00040 |
| [19] |
(a) Dudnik, A. S.; Aldrich, T. J.; Eastham, N. D.; Chang, R. P.; Facchetti, A.; Marks, T. J. J. Am. Chem. Soc. 2016, 138, 15699.
doi: 10.1021/jacs.6b10023 |
|
(b) Wakioka, M.; Ishiki, S.; Ozawa, F. Macromolecules 2015, 48, 8382.
doi: 10.1021/acs.macromol.5b01822 |
|
|
(c) Aldrich, T. J.; Dudnik, A. S.; Eastham, N. D.; Manley, E. F.; Chen, L. X.; Chang, R. P. H.; Melkonyan, F. S.; Facchetti, A.; Marks, T. J. Macromolecules 2018, 51, 9140.
doi: 10.1021/acs.macromol.8b02297 |
|
| [20] |
(a) Zhang, C.; Matos, T.; Li, R.; Sun, S.-S.; Lewis, J. E.; Zhang, J.; Jiang, X. Polymer Chemistry 2010, 1, 663.
doi: 10.1039/b9py00324j |
|
(b) Wang, M.; Wang, H.; Yokoyama, T.; Liu, X.; Huang, Y.; Zhang, Y.; Nguyen, T. Q.; Aramaki, S.; Bazan, G. C. J. Am. Chem. Soc. 2014, 136, 12576.
doi: 10.1021/ja506785w |
|
| [21] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery Jr, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16, Wallingford CT, 2016.
|
| [22] |
Kim, J. S.; Fei, Z.; Wood, S.; James, D. T.; Sim, M.; Cho, K.; Heeney, M. J.; Kim, J.-S. Adv. Energy Mater. 2014, 4, 1400527.
doi: 10.1002/aenm.v4.18 |
| [23] |
Más-Montoya, M.; Janssen, R. A. J. Adv. Funct. Mater. 2017, 27, 1605779.
doi: 10.1002/adfm.v27.16 |
| [24] |
Kang, S. H.; Lee, D.; Kim, H.; Choi, W.; Oh, J.; Oh, J. H.; Yang, C. ACS Appl. Mater. Interfaces 2021, 13, 52840.
doi: 10.1021/acsami.1c14945 |
| [25] |
(a) Tu, Q.; Ma, Y.; Zhou, X.; Ma, W.; Zheng, Q. Chem. Mater. 2019, 31, 5953.
doi: 10.1021/acs.chemmater.9b02355 |
|
(b) Huang, H.; Bin, H.; Peng, Z.; Qiu, B.; Sun, C.; Liebman-Pelaez, A.; Zhang, Z.-G.; Zhu, C.; Ade, H.; Zhang, Z.; Li, Y. Macromolecules 2018, 51, 6028.
doi: 10.1021/acs.macromol.8b01036 |
|
| [26] |
Brown, A. R.; Jarrett, C. P.; de Leeuw, D. M.; Matters, M. Synth. Met. 1997, 88, 37.
doi: 10.1016/S0379-6779(97)80881-8 |
| [27] |
Tsurui, K.; Murai, M.; Ku, S.-Y.; Hawker, C. J.; Robb, M. J. Adv. Funct. Mater. 2014, 24, 7338.
doi: 10.1002/adfm.v24.46 |
| [28] |
Wang, Y.; Liu, Y.; Chen, S.; Peng, R.; Ge, Z. Chem. Mater. 2013, 25, 3196.
doi: 10.1021/cm401618h |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||