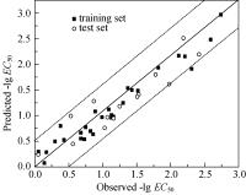
The toxicity of nitroaromatic compounds to Tetrahymena pyriformis was studied by quantitative structure activity relationship (QSAR). The partial least squares approach was empolyed to establish a quantitative model which included 19 quantum-chemical and physical-chemical parameters calculated from 42 nitroaromatic compounds based on the semi-empirical AM1 method, and included the toxicity data of Tetrahymena pyriformis and part of molecular structures reported in literatures. The reliability of the model was evaluated and compared with other reported models. The toxicity of nitroaromatic compounds was also analyzed. Results showed that the model had a good predictive capability (R2=0.946, =0.921, P=5.5×10-18, F=453.53, SD=0.171), indicating higher accuracy achieved as compared to other models. The parameters of ELUMO, a, , ET, MW, CSV and MR played important roles in the toxicity inhibition of nitroaromatic compounds, among which ELUMO, ET and △E had negative correlations with the toxicity, while a, , , △Hf, MW, CSV and MR had positive correlations with the toxicity. The difference in the contribution to the Tetrahymena pyriformis toxicity by ET and △Hf is possibly caused by different types of mechanisms responsible for their toxicity to the nitroaromatic compounds.
CHEN Xue-Yong
,
WEI Chao-Hai
,
DENG Xiu-Qiong
,
XIA Fang
,
YU Xu-Biao
. An Analysis of the Toxicity of Nitroaromatic Compounds to Tetrahymena pyriformis Using the Quantitative Structure Activity Relationship Method[J]. Acta Chimica Sinica, 2011
, 69(21)
: 2618
-2626
.
DOI: 10.6023/A1101082B


