

K3PO4-Catalyzed Regiospecific Aminobromination of β-Nitrostyrene Derivatives with acetamide and NBS
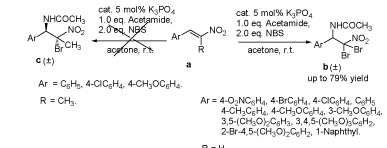
CHEN Zhan-Guo , ZHOU Ji-Mei , WANG Yun , LI Wen-Li . K3PO4-Catalyzed Regiospecific Aminobromination of β-Nitrostyrene Derivatives with acetamide and NBS[J]. Acta Chimica Sinica, 2011 , 69(23) : 2851 -2858 . DOI: 10.6023/A1103229
/
| 〈 |
|
〉 |