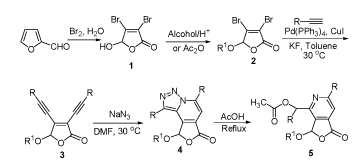
A novel tandem cyclization of 2(5H)-furanone derivatives containing enediyne structure with sodium azide giving rise to serial novel 1,2,3-triazole polycyclic compounds was accomplished. Under the optimized conditions, including 1.5 equiv. sodium azide, DMF as the solvent, the tandem reaction at 30 ℃ for 48 h gave the 2(5H)-furanone derivatives containing tricyclic structure in moderate yields (42%~62%). The tricyclic fused 2(5H)-furanone derivatives with 1,2,3-triazole structure could be converted into fused pyridine derivatives with high yields (94%~96%). The structure of all fused 2(5H)-furanone derivatives was confirmed by IR, 1H NMR, 13C NMR, MS and elemental analysis. And the possible reaction mechanism of the tandem cyclization was proposed. This tandem reaction has the advantages of easy operation, mild reaction condition and without the addition of catalyst. Moreover, this method was a convenient and simple path for the synthesis of the tricyclic fused 2(5H)-furanone derivatives containing 1,2,3-triazole.
LI Jian-Xiao
,
WANG Chao-Yang
,
XUE Fu-Ling
,
LUO Shi-He
. Synthesis of Novel Tricyclic Fused 2(5H)-Furanone Derivatives with 1,2,3-Triazole Structure[J]. Acta Chimica Sinica, 2011
, 69(23)
: 2835
-2842
.
DOI: 10.6023/A1106131


