

Preparation and pH-Sensitive Drug Delivery Study of mPEGpoly(Imidazole Propyl-Asparagine)-poly(L-Alanine)
Received date: 2011-09-08
Revised date: 2011-11-30
Online published: 2012-02-25
Supported by
Project supported by the Natural Science Foundation of Tianjin (Nos.09JCYBJC03400, 10JCYBJC26800);the Ph.D. Programs Foundation for New Teachers of Education Ministry of China (No.20090031120012) and Fundamental Research Funds for the Central Universities.
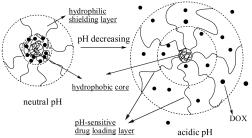
Key words: poly(aspartic acid); imidazole; DOX; pH-responsive; drug delivery
YU Shu-Fang , GU Xin , WU Guo-Lin , WANG Zheng , WANG Yi-Nong , GAO Hui , MA Jian-Biao . Preparation and pH-Sensitive Drug Delivery Study of mPEGpoly(Imidazole Propyl-Asparagine)-poly(L-Alanine)[J]. Acta Chimica Sinica, 2012 , 70(02) : 177 -182 . DOI: 10.6023/A1109081










/
| 〈 |
|
〉 |