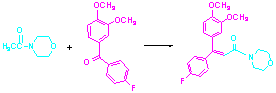
Flumorph is a new fungicide, thermal hazard study and kinetic analysis for the process will solve engineering problems and ensure production safety. The thermostability of main materials is tested by using differential scanning calorimetry-thermogravimetric analyzer (DSC-TG). The reaction thermal behavior is studied by using reaction calorimeter (RC1), and the kinetic study of the reaction is carried out in this paper. The conclusion of this study is that the endothermic decomposition temperature of main intermediate of (3,4-dimethoxyphenyl)(4-fluorophenyl)methanone is 559.3 K. The exothermic decomposition temperature of N-acetylmorpholine is 478.2 K and the endothermic decomposition temperature of flumorph is 638.6 K. The exothermic energy of the reaction for synthesis of flumorph is 15.44 kJ/mol, and the adiabatic temperature rise is 9.1 K. This process is with lower thermal hazard. The reaction kinetic equation is rA=kcAa=8.34×10-3CA0.57, and the reaction order is 0.57 for (3,4-dimethoxyphenyl)(4-fluorophenyl)- methanone.
CHENG Chun-Sheng
,
QIN Fu-Tao
,
WEI Zhen-Yun
,
REN Zhong-Bao
,
MING Xu
. Thermal Hazard and Kinetic Study for Synthesis Process of Flumorph[J]. Acta Chimica Sinica, 2012
, 70(10)
: 1227
-1231
.
DOI: 10.6023/A1112122
1 Li, Z.-C.; Liu, C.-L.; Liu, W.-C. CN 1155977 1997 [Chem. Abstr. 1999, 132, 9930].
2 Liu, W.-C.; Liu, C.-L. Agrochemicals 2001, 41(1), 8 (in Chinese). (刘武成, 刘长令, 农药, 2001, 41(1), 8.)
3 Sun, D.-Q.; Tian, A.-J.; Yang, Y. Modern Agrochem. 2009,8(2), 32 (in Chinese). (孙德群, 田爱俊, 杨越, 现代农药, 2009, 8(2), 32.)
4 Gygax, R. Chem. Eng. Sci. 1988, 43(8), 1759.
5 Stoessel, F.; Ubrich, O. J. Therm. Anal. Calorim. 2001,64(1), 61.
6 Stoessel, F. Thermal Safety of Chemical Processes-Risk Assessment and Process Design, Berlin, Wiley-VCH, 2008, p.63.
7 Cheng, C.-S.; Qin, F.-T.; Wei, Z.-Y. Chemical Safety Production and Reaction Risk Assessment, Chemical Industry Press, Beijing, 2011, pp. 55~63 (in Chinese). (程春生, 秦福涛, 魏振云, 化工安全生产与反应风险评 估, 化学工业出版社, 北京, 2011, pp. 55~63.)
8 Stoessel, F. Chem. Eng. Prog. 1993, 89(10), 68.
9 Stoessel, F. Chem. Eng. Prog. 1995, 91(9), 46.


