

Possible Reaction between Titanium(IV) and Hydrogen Peroxide in Acidic Condition: An ab-initio Study on Its Mechanism
Received date: 2012-05-07
Online published: 2012-08-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 50871124), the Open-End Fund for the Valuable and Precision Instruments of Central South University (CSUZC2012023) and the Open Fund of State Key Laboratory of Powder Metallurgy.
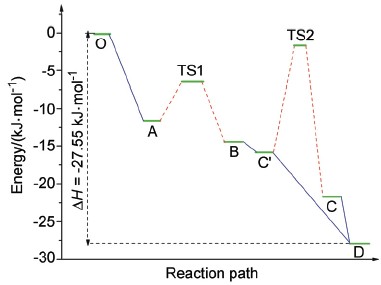
The reaction between titanyl hydroxy and titanium(IV) has long been interested in and studied as its value in catalysts, analytic chemistry and synthesis of novel ceramic materials. Nevertheless, the possible reaction mechanism and its final products have never been concluded under different experimental conditions. Even in some particular condition i.e. pH<1, different products had been proposed such as Ti(O2)2+ and Ti(O2)(OH). In this article, a possible reaction mecha-nism has been proposed based on first-principles calculations in order to explain its experimental phenomenon under specific condition(s) (pH<1). The calculation is carried out by the combination of B3LYP functional and 6-311G+(3df,2p) basis set. As to estimate the solvent effect, the IEF-PCM model was also introduced in the calculation. Based on the calculation level mentioned above, the reaction profile and heat were figured out, and heat of reaction is comparable to the experimental data (27 kJ/mol vs. 24 kJ/mol). The rate limiting steps are also identified by checking the transformation of molecular orbitals before and after triatomic ring annulation reaction and hydrogen (H+) transfer reaction. In the annulation reaction, the complicated interaction between Ti's 3d orbitals and O's 2p orbitals acts crucial role in the transformation of bonding types into triatomic ring. In the hydrogen (H+) transfer reaction, the vacant orbital 1s of hydrogen (H+) attacks the HOMO of the titanyl peroxo complex to form new O—H bond while breaking the old O—H bond in the complex. The two steps reaction model fits well with pseudo-first-order hypothesis of the general dynamics of the reaction and the deviation of the general reaction dynamics as well, which supports the thermochemistry results made by first principles calculation. We hope this possible mechanism not only explains the reaction products under the specific condition, but also can be extended to other transition metals' reactions with peroxo complex and find its value in the synthesis of epoxidation catalysts and ceramics.
Key words: titanium(IV); hydrogen peroxide; B3LYP; molecular orbital; reaction dynamics
Tang Kan , Tao Huijin , Jiang Xinyu . Possible Reaction between Titanium(IV) and Hydrogen Peroxide in Acidic Condition: An ab-initio Study on Its Mechanism[J]. Acta Chimica Sinica, 2012 , 70(19) : 2091 -2096 . DOI: 10.6023/A12050182
/
| 〈 |
|
〉 |