

Studies on the Reactivity of Migrating Group in [2,3]-Sigmatropic Rearrangement of Sulfur Ylides
Received date: 2012-06-24
Online published: 2012-09-03
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21072009, 21172005), National Basic Research Program of China (973 Program, No. 2009CB825300), The Project Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
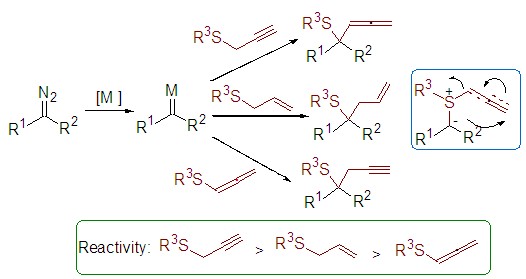
The [2,3]-sigmatropic rearrangement of sulfur ylides is unique and useful reaction in organic synthesis. In this study, the reactivity of sulfides containing three different migrating groups (propargyl, allyl, and allenyl) in [2,3]-sigmatropic rearrangement of sulfur ylides has been compared. The competition reactions of phenylethyldiazoacetate with sulfides through sulfonium ylide [2,3]-sigmatropic rearrangement are designed under Rh(II)- or Cu(I)-catalyzed reaction conditions. Both intra- and intermolecular competitions of sulfides bearing two different migrating groups have been carried out. The ratio of products has been determined by 1H NMR in order to compare the reactivity of different sulfides bearing allyl, propargyl or allenyl groups. Obvious disparity of the reactivity of these sulfides in [2,3]-sigmatropic rearrangement has been observed. Experimental data indicate that the tendency of preferential [2,3]-sigmatropic rearrangement has the following order: propargyl sulfide>allyl sulfide>allenyl sulfide. Catalysts such as Rh2(OAc)4, Rh2(O2CCF3)4, and Cu(CH3CN)4PF6 ligated with a series of diimine ligands have been investigated for these reactions. Rh(II) complexes are found more efficient than Cu(I) complexes, and Rh2(O2CCF3)4 is more efficient than Rh2(OAc)4. The efficiency of different catalytic system has been explained based on the proposed reaction mechanism. The reaction catalyzed by Rh(II) complexes is suggested to be different from that catalyzed by Cu(I) complexes. In the case of Cu(I)-catalyzed reaction, Cu(I)-bonded sulfur ylide is considered as the predominant intermediate, while the [2,3]-sigmatropic rearrangement is suggested to proceed through free ylide. Both steric hindrance and electronic properties of ligands influence the ratio and selectivity in Cu(I)-catalyzed reactions. This study provides useful information for further investigation of [2,3]-sigmatropic rearrangement of sulfur ylides.
Li Yuye , Huang Zhongxing , Xu Peng-Fei , Zhang Yan , Wang Jianbo . Studies on the Reactivity of Migrating Group in [2,3]-Sigmatropic Rearrangement of Sulfur Ylides[J]. Acta Chimica Sinica, 2012 , 70(19) : 2024 -2028 . DOI: 10.6023/A12060337
[1] (a) Baldwin, J. E.; Hackler, R. E.; Kelley, D. P. J. Am. Chem. Soc. 1968, 90, 4758; (b) Blackburn, G. M.; Ollis, W. D.; Smith, C.; Suth-erland, I. O. J. Chem. Soc. Chem. Commun. 1968, 186; (c) Baldwin, J. E.; Hackler, R. E.; Kelley, D. P. J. Chem. Soc. Chem. Commun. 1968, 537.
[2] For selected reviews, see: (a) Reggelin, M. Top. Curr. Chem. 2007, 275, 1; (b) Braverman, S.; Cherkinsky, M. Top. Curr. Chem. 2007, 275, 67; (c) Ando, W.; Matsuyama, H. Nitrogen, Oxygen, and Sulfur Ylide Chemistry, Ed.: Clark, J. S., Oxford University Press, Oxford, 2002, p. 163; (d) Zhang, Y.; Wang, J. Coord. Chem. Rev. 2010, 254, 941.
[3] For selected recent reports, see: (a) Ma, M.; Peng, L.; Li, C.; Zhang, X.; Wang, J. J. Am. Chem. Soc. 2005, 127, 15016; (b) Liao, M.; Wang, J. Green Chem. 2007, 9, 184; (c) Zhou, C.-Y.; Huang, J.-S.; Che, C.-M. Synlett 2010, 18, 2681; (d) Vorobyeva, D. V.; Mailyan, A. K.; Peregudov, A. S.; Karimova, N. M.; Vasilyeva, T. P.; Bush-marinov, I. S.; Bruneau, C.; Dixneuf, P. H.; Osipov, S. N. Tetrahe-dron 2011, 67, 3524; (e) Raghavan, S.; Kumar, V. V.; Chowhan, L. R. Synlett 2010, 12, 1807; (f) McMillen, D. W.; Varga, N.; Reed, B. A.; King, C. J. Org. Chem. 2000, 65, 2532; (g) Zhang, X.; Qu, Z.; Ma, Z.; Shi, W.; Jin, X.; Wang, J. J. Org. Chem. 2002, 67, 5621; (h) Zhang, X.; Ma, M.; Wang, J. Tetrahedron: Asymmetry 2003, 14, 891.
[4] (a) Trost, B. M.; Melvin, L. S. Jr. Sulfur Ylides, Academic Press, New York, 1975, Chapter 7; (b) Briere, J.-F.; Metzner, P. Organo-sulfur Chemistry in Asymmetric Synthesis, Eds.: Toru, T.; Bolm, C. Wiley-VCH, Weinheim, 2008, pp. 179~208.
[5] Ando, W. Acc. Chem. Res. 1977, 10, 179.
[6] Related reviews: (a) Ye, T.; McKervey, M. A. Chem. Rev. 1994, 94, 1091; (b) Padwa, A.; Weingarten, M. D. Chem. Rev. 1996, 96, 223. (c) Li, A.-H.; Dai, L.-X.; Aggarwal, V. K. Chem. Rev. 1997, 97, 2341; (c) Padwa, A. Chem. Commun. 1998, 1417; (d) Doyle, M. P.; McKervey, M. A.; Ye, T. Morden Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley-Interscience, New York, 1998; (e) Padwa, A. J. Organomet. Chem. 2001, 3, 617. (e) Mehta, G.; Muthusamy, S. Tetrahedron 2002, 58, 9477; (f) Aggarwal, V. K.; Winn, C. L. Acc. Chem. Res. 2004, 37, 611; (g) Wee, A. G. H. Curr. Org. Synth. 2006, 3, 499; (h) Hodgson, O. M.; Pierard, F. Y. T. M.; Stupple, P. A. Chem. Soc. Rev. 2001, 30, 50.
[7] (a) Kamikawa, K.; Tachibana, A.; Shimizu, Y.; Uchida, K.; Furusyo, M.; Uemura, M. Tetrahedron 2006, 62(5), 922; (b) Kato, Y.; Miki, K.; Nishino, F.; Ohe, K.; Uemura, S. Org. Lett. 2003, 5, 2619.
[8] (a) Kirmse, W.; Kapps, M. Chem. Ber. 1968, 101, 994; (b) Doyle, M. P.; Griffin, J. H.; Chinn, M. S.; van Leusen, D. J. Org. Chem. 1984, 49, 1917.
[9] For selected reviews, see: (a) Franssen, N. M. G.; Walters, A. J. C., Reek, J. N. H.; de Bruin, B. Catal. Sci. Technol. 2011, 1(2), 153; (b) Barluenga, J.; Valdés, C. Angew. Chem. Int. Ed. 2011, 50, 7486; (c) Shao, Z.; Zhang, H. Chem. Soc. Rev. 2012, 41, 560.
[10] Li, Y.; Huang, Z.; Wu, X.; Xu P.-F.; Jin, J.; Zhang, Y.; Wang, J. Tetrahedron 2012, 68(26), 5234.
[11] (a) Gulea, M.; Marchand, P.; Masson, S.; Saquet, M.; Collignon, N. Synthesis 1998, 11, 1635; (b) Khanova, M. D.; Sultanova, R. M.; Ra?kov, R. R.; Baykova, I. P.; Biglova, R. Z.; Dokichev, V. A.; Tomilov, Y. V. Russ. Chem. Bull. 2008, 57, 617.
[12] (a) Moore, J. D.; Sprott, K. T.; Hanson, P. R. Synlett 2001, 5, 605; (b) Stepakov, A. V.; Molchanov, A. P.; Magull, J.; Vidovic, D.; Starova, G. L.; Kopf, J.; Kostikov, R. R. Tetrahedron 2006, 62, 3610; (c) Pang, W.; Zhu, S.; Jiang, H.; Zhu, S. Tetrahedron 2007, 63, 4543.
[13] (a) Carter, D. S.; Van Vranken, D. L. Org. Lett. 2000, 2, 1303; (b) Aviv, I.; Gross, Z. Chem. Eur. J. 2008, 14, 3995.
[14] (a) Zhou, C.-Y.; Yu, W.-Y.; Chan, P. W. H.; Che, C.-M. J. Org. Chem. 2004, 69, 7072; (b) Xiao, Q.; Wang, J. Acta Chim. Sinica 2007, 65, 1733 (肖卿, 王剑波, 化学学报, 2007, 65, 1733.)
[15] Davies, P. W.; Albrecht, S. J.-C. Chem. Commun. 2008, (2), 238.
[16] Davies, P. W.; Albrecht, S. J.-C.; Assanelli, G. Org. Biomol. Chem. 2009, 7, 1276.
[17] Kunishima, M.; Nakata, D.; Goto, C.; Hioki, K.; Tani, S. Synlett 1998, 12, 1366.
[18] Greenman, K. L.; Carter, D. S.; Van Vranken, D. L. Tetrahedron 2001, 57, 5219.
[19] (a) Fukuda, T.; Katsuki, T. Tetrahedron Lett. 1997, 38, 3435; (b) Fukuda, T.; Irie, R.; Katsuki, T. Tetrahedron 1999, 55, 649.
[20] Crich, D.; Zou, Y.; Brebion, F. J. Org. Chem. 2006, 71, 9172.
[21] (a) Nakai, T.; Mikami, K.; Lee, S. O.; Masuyama, Y. Abstracts of Papers, American Chemical Society/Chemical Society of Japan Chemical Congress, Honolulu, American Chemical Society, Wash-ington, DC, 1979; (b) ORGN 349. Cf.: Mikami, K. Ph.D. Disserta-tion, Tokyo Institute of Technology, 1982, p. 58.
[22] Davies, P. W.; Albrecht, S. J.-C.; Assanelli, G. Org. Biomol. Chem. 2009, 7, 1276.
[23] Liang, Y.; Zhou, H.; Yu, Z.-X. J. Am. Chem. Soc. 2009, 131, 17783.
/
| 〈 |
|
〉 |