

Performance Study of Water/Alcohol Soluble Polymer Interface Materials in Polymer Optoelectronic Devices
Received date: 2012-10-15
Online published: 2012-12-07
Supported by
Project supported by the Ministry of Science and Technology (Nos. 2009CB623601 and 2009CB930604), the Natural Science Foundation of China (Nos. 21125419, 50990065, 51010003 and 51073058) and Guangdong Natural Science Foundation (No. S2012030006232).
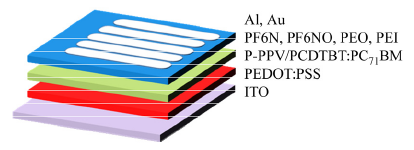
Four different water/alcohol soluble polymers, poly[9,9-bis(6-(N,N-diethylamino)-hexyl N-oxide)fluorene] (PF6NO), poly[9,9-bis(6-(N,N-diethylamino)-hexyl)fluorene] (PF6N), polyethylene oxide (PEO), polyethylene imine (PEI) were used as interlayer materials in polymer light-emitting diodes (PLEDs) and polymer solar cells (PSCs) with device structures of indium tin oxide (ITO)/poly(3,4-ethylenedioxylenethiophene):poly(styrenesulphonic acid) (PEDOT:PSS)/Active Layer/Interlayer/Al (or Au), where the green light-emitting polymer poly-[2-(4-(3',7'-dimethyloctyloxy)-phenyl)-p-phenylene vinylene] (P-PPV) and photovoltaic material poly-[N-9'-hepta-decanyl-2,7-carbazole-alt-5,5-(4',7'-di-2-thienyl-2',1',3-benzothiadiazole) (PCDTBT):[6,6]-phenyl C 71-butyric acid methyl ester (PC71BM) were used as the light emitting layer and light absorption layers for PLEDs and PSCs, respectively. The relationships between chemical structure and optoelectronic properties of the polymer interlayer materials were systematically investigated. Photovoltaic measurement were used to determine the built-in potential across the devices and consequently to understand the interface modification abilities of these materials. It was found that the devices based on conjugated interlayer materials exhibited larger open circuit voltages than those of devices based on non-conjugated interlayer materials, which indicates that the conjugated interlayer materials lead to lower energetic barriers for electron. Device studies showed that the conjugated interlayer materials PF6NO and PF6N exhibited much better device performance both in PLEDs and PSCs compared to the non-conjugated interlayer materials PEO and PEI, which was consistent with the photovoltaic measurement results. PLEDs studies indicated that the devices based on non-conjugated interlayer materials PEO and PEI exhibited dramatically decreased efficiencies when the interlayers?? thicknesses are more than 20 nm, while the devices based on conjugated interlayer materials PF6NO and PF6N maintained good performances. Moreover, both PLEDs and PSCs device studies indicated that the conjugated interlayer materials show a wide range of adaptability, which work well for both Al and Au electrode devices, while the non-conjugated interlayer materials only work well for the Al electrode devices. Our results indicated that besides the highly polar side chain groups, the conjugated interlayer materials?? main chains also play an important role on their excellent interfacial modification ability, which lead to easily electron transporting and wide range of adaptability.
Zhang Kai , Guan Xing , Huang Fei , Cao Yong . Performance Study of Water/Alcohol Soluble Polymer Interface Materials in Polymer Optoelectronic Devices[J]. Acta Chimica Sinica, 2012 , 70(24) : 2489 -2495 . DOI: 10.6023/A12100777
/
| 〈 |
|
〉 |