

Mesoscopic Simulation Studies on the Self-assembly of Pluronic at Oil/Water Interface
Received date: 2012-12-10
Online published: 2013-01-11
Supported by
Project supported by the National Natural Science Foundation of China (No. 20803069) and the Science Foundation for Excellent Middle & Young Scientist of Shandong Province (No. BS2010CL050).
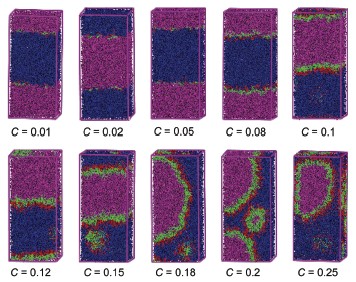
The dissipative particle dynamics (DPD) simulation method was used to simulate the self-assembly behavior of three triblock copolymers, EO106PO70EO106, EO80PO30EO80 and EO13PO30EO13, at the oil/water interface. Gaussian chain is used to represent the three triblock copolymers. The effects of molecular weight, PO/EO ratio and oil/water volume ratio on the aggregation behavior of the copolymer were discussed in our simulation. The simulation results indicate that the triblock copolymer aggregate at the oil/water interface at lower concentration. The PEO groups of copolymer chains are immersed in the water phase while the PPO groups are located close to the oil phase. With the concentration of copolymer increasing, some copolymer chains begin stretch into the bulk. Copolymers can self-assemble into micelles in the water phase when the surface is saturated. The PEO groups are in the surface of micelles and some oil beads in the core, and this is in good agreement with the experimental results concerning their structures. In addition, molecular weight, and the ratio of PO/EO and oil/water have great effect on the interfacial properties (interfacial density, interfacial thickness and interfacial tension) and structural properties of the triblock copolymers. We found that the oil/water/copolymer system has lower interfacial tension and higher interfacial thickness when the copolymer has higher PO/EO ratio and larger molecular weight. If the value of PO/EO ratio is similar, the interfacial tension and interfacial thickness increases with molecular weight. Though the PO/EO ratio of F68 is lower than F127, the arrangement of F68 chains at the interface is more compact because of its lower molecular weight. With oil/water ratio increasing, O/W type emulsion is gradually transformed to a W/O type emulsion for the oil/water/copolymer systems. Furthermore, the effect of oil/water ratio on the self-assembly behavior of block copolymer is related to its molecular weight. Significant effect is observed for larger molecular weight of triblock copolymers (F127 and F68), but little effect on L64. Our simulation results show that DPD simulation is a valuable tool to supplement the experimental study on the micro-structural properties of Pluronic copolymers.
Sun Nuannuan , Li Yiming , Wang Dongxiang , Bao Mutai , Tong Linjuan . Mesoscopic Simulation Studies on the Self-assembly of Pluronic at Oil/Water Interface[J]. Acta Chimica Sinica, 2013 , 71(02) : 186 -192 . DOI: 10.6023/A12121023
[1] Liang, X.-F.; Guo, C.; Liu, Q.-F.; Liu, H.-Z. J. Chem. Ind. Eng. 2010, 61, 1693. (梁向峰, 郭晨, 刘庆芬, 刘会洲, 化工学报, 2010, 61, 1693.)
[2] Su, Y.-L.; Guo, C.; Liu, H.-Z. J. Chem. Ind. Eng. 2003, 54, 489. (苏延磊, 郭晨, 刘会洲, 化工学报, 2003, 54, 489.)
[3] Lin, C.-Y.; Qiu, Y.; Jiang, L.-Q.; Zhao, J.-X. J. Fuzhou Univ. (Nat. Sci. Ed.) 2000, 28, 77. (林翠英, 邱羽, 江琳沁, 赵剑曦, 福州大学学报(自然科学版), 2000, 28, 77.)
[4] Won, Y. Y.; Brannan, A. K.; Davis, H. T.; Bates, F. S. J. Phys. Chem. B 2002, 106, 3354.
[5] Nolan, S. L.; Philips, R. J.; Cotts, P. M.; Dungan, S. R. J. Colloid Interface Sci. 1997, 191, 291.
[6] Aswal, V. K.; Goyal, P. S.; Kohlbrecher, J.; Bahadur, P. Chem. Phys. Lett. 2001, 349, 458.
[7] Schmidt, G.; Richtering, W.; Lindner, P.; Alexandridis, P. Macromolecules 1998, 31, 2293
[8] Yuan, S.-L.; Wu, R.; Cai, Z.-T. Acta Phys.-Chim. Sin. 2004, 20, 811. (苑世领, 吴锐, 蔡政亭, 物理化学学报, 2004, 20, 811.)
[9] Chen, Y.; Zhong, J.; Huang, W.-Q. Chem. J. Chin. Univ. 2010, 31, 1827. (陈燕, 钟璟, 黄维秋, 高等学校化学学报, 2010, 31, 1827.)
[10] Guo, S.-L.; Hou, T.-J.; Xu, X.-J. Comput. Appl. Chem. 2002, 19, 298. (郭森立, 侯廷军, 徐筱杰, 计算机与应用化学, 2002, 19, 298.)
[11] Loos, K.; Müller, A. H. E. Biomacromolecules 2002, 3, 368.
[12] Du, H.-B.; Zhu, J.-T.; Jiang, W. J. Phys. Chem. B 2007, 111, 1938.
[13] Goldmints, I.; Yu, G.; Booth, C.; Smith, K. A.; Hatton, T. A. Langmuir 1999, 15, 1651.
[14] Kloxin, C. J.; van Zanten, J. H. Macromolecules 2010, 43, 2084.
[15] du Sart, G. G.; Vukovic, I.; van Ekenstein, G. A. Macromolecules 2010, 43, 2970.
[16] Li, C.; Gao, Y.; Zhang, J.-J.; Zhu, J.-B.; Wu, L.-H. Acta Chim. Sinica 2011, 69, 1503. (李超, 高缘, 张建军, 朱家壁, 吴莲花, 化学学报, 2011, 69, 1503.)
[17] Hoogerbrugge, P. J.; Koelman, J. M. V. A. Eur. Lett. 1992, 19, 155.
[18] Koelman, J. M. V. A.; Hoogerbrugge, P. J. Eur. Lett. 1993, 21, 363.
[19] Groot, R. D. Langmuir 2000, 16, 7493.
[20] Zhang, X.-Q.; Yuan, S.-L.; Xu, G.-Y.; Liu, C.-B. Acta Phys.-Chim. Sin. 2007, 23, 139. (张秀青, 苑世领, 徐桂英, 刘成卜, 物理化学学报, 2007, 23, 139.)
[21] Alexandridis, P.; Holzwarth, J. F.; Hatton, T. A. Macromolecules 1994, 27, 2414.
[22] Wanka, G.; Hoffmann, H.; Ulbricht, W. Macromolecules 1994, 27, 4145.
[23] Chu, B. Langmuir 1996, 11, 414.
[24] Luo, M.-X.; Dai, L.-L. J. Phys.: Condens. Matter 2007, 19, 375109.
/
| 〈 |
|
〉 |