

Interrupted Fisher Indole Synthesis and Its Applications to Alkaloid Synthesis
Received date: 2013-01-05
Online published: 2013-01-25
Supported by
Project supported by the National Basic Research Program (973 Program) of China (No. 2013CB836900), the Natural Science Foundation of China (Nos.21172235, 21222202, and 21290180), Shanghai Pujiang Plan (No. 12PJ1410800) and the Chinese Academy of Sciences.
Fisher indole synthesis is an important method in organic synthesis. Recently, Garg and Liang developed interrupted Fisher indole synthesis and applied it in the synthesis of a series of natural products and natural product-like compounds. Here we highlight their recent progress under this topic.
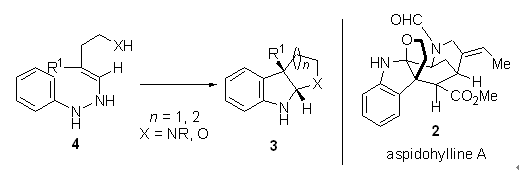
Li Sena , Han Jing , Li Ang . Interrupted Fisher Indole Synthesis and Its Applications to Alkaloid Synthesis[J]. Acta Chimica Sinica, 2013 , 71(03) : 295 -298 . DOI: 10.6023/A13010018
[1] Bandini, M.; Eichholzer, A. Angew. Chem., Int. Ed. 2009, 48, 9608.
[2] Schammel, A. W.; Garg, N. K. Org. Lett. 2009, 11, 3458.
[3] (a) Song, H.; Yang, J.; Chen, W.; Qin, Y. Org. Lett. 2006, 8, 6011;
(b) Yang, J.; Wu, H. X.; Shen, L. Q.; Qin, Y. J. Am. Chem. Soc.2007, 129, 13794;
(c) Shen, L. Q.; Zhang, M.; Wu, Y.; Qin, Y.Angew. Chem., Int. Ed. 2008, 47, 3618;
(d) Zhang, M.; Huang, X.P.; Shen, L. Q.; Qin, Y. J. Am. Chem. Soc. 2009, 131, 6013.
[4] (a) Jones, S. B.; Simmons, B.; MacMillan, D. W. C. J. Am. Chem.Soc. 2009, 131, 13606;
(b) Jones, S. B.; Simmons, B.; Mastracchio,A.; MacMillan, D. W. C. Nature 2011, 475, 183.
[5] (a) Zuo, Z.; Xie, W.; Ma, D. J. Am. Chem. Soc. 2010, 132, 13226;
(b) Zuo, Z.; Ma, D. Angew. Chem., Int. Ed. 2011, 50, 12008;
(c) Zi,W.; Xie, W.; Ma, D. J. Am. Chem. Soc. 2012, 134, 9126;
(d) Fan, F.;Xie, W.; Ma, D. Org. Lett. 2012, 14, 1405.
[6] Zhan, F.; Liang, G. Angew. Chem., Int. Ed. 2013, 52, 1266.
[7] (a) Schammel, A. W.; Boal, B. W.; Zu, L.; Mesganaw, T.; Garg, N.K. Tetrahedron 2010, 66, 4687;
(b) Çelebi-Ölçüm, N.; Boal, B. W.;Huters, A. D.; Garg, N. K.; Houk, K. N. J. Am. Chem. Soc. 2011,133, 5752;
(c) Schammel, A. W.; Chiou, G.; Garg, N. K. J. Org.Chem. 2012, 77, 725;
(d) Schammel, A. W.; Chiou, G.; Garg, N. K.Org. Lett. 2012, 13, 4556.
[8] Subramaniam, G.; Hiraku, O.; Hayashi, M.; Koyano, T.; Komiyama,K.; Kam, T. S. J. Nat. Prod. 2007, 70, 1783.
[9] Zu, L.; Boal, B. W.; Garg, N. K. J. Am. Chem. Soc. 2011, 133, 8877.
/
| 〈 |
|
〉 |