

Novel PVA/SiO2 Alkaline Micro-porous Polymer Electrolytes for Polymer Ni—MH Batteries
Received date: 2012-11-05
Online published: 2013-02-01
Supported by
Project supported by National Natural Science Foundation of China (Nos. 51171079, 51071085), Specialized Research Fund for the Doctoral Program of High Education (No. 20093221110008) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
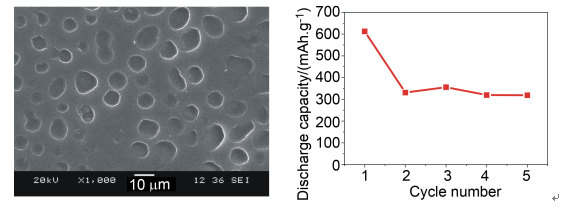
New poly(vinyl alcohol)/silica (designated as PVA/SiO2) alkaline micro-porous polymer electrolytes (AMPEs) were prepared by soaking PVA/SiO2 micro-porous composite membranes, obtained by solution casting of PVA/PEG/SiO2 membrane in acetone solution, into an electrolyte solution of 6 mol/L KOH aqueous solution. The morphology and structure of PVA/SiO2 composite polymer membranes were characterized by scanning electron microscopy (SEM) and X-Ray diffraction (XRD). The SEM photographs showed that the nano-SiO2 filler content was a crucial issue for the well-dispersed and optimal-sized pores which could storage charge carrier durably. Meanwhile, the crystalline of PVA decreased effectively for a large number of crystal defects and free volume appeared in the interface of inorganic particles and polymer for the addition of nano-SiO2 filler. The electrochemical properties of the AMPEs were measured by the alternating current impedance (AC impedance) and the cyclic voltammetry (CV) techniques. The results indicated that the PVA/SiO2 AMPEs containing 5 ω nano-SiO2 filler exhibited good performances at room temperature, such as 1.62×10-2S·cm-1for ionic conductivity and 2.20 V for electrochemical stability window. What's more, we used the gravimetric method to obtain the electrolyte uptake of various PVA/SiO2 composite micro-porous polymer membranes. From the data, we learned that the maximum electrolyte uptake could reach to 102.7% and it had very relevance to the size of pores in PVA/SiO2 composite polymer membranes, and then influenced the ionic conductivity. Each polymer Ni-MH battery was assembled by three parts: the new AMPE, Mg-based hydrogen storage alloy and the commercial sintered Ni(OH)2/NiOOH electrode, in which each part did for electrolyte and diaphragm, negative electrode and positive electrode, respectively. The cycle experiments of the batteries exhibited a high first-cycle discharge capacity of 613 mAh·g-1 and stable discharge capacities about 330 mAh·g-1 for the following 5 cycles. The results encouraged that the novel AMPEs are prospective for the applications of polymer electrolyte in Ni-MH battery field.
Lu Xia , Wu Renxiang , Li Bobo , Zhu Yunfeng , Li Liquan . Novel PVA/SiO2 Alkaline Micro-porous Polymer Electrolytes for Polymer Ni—MH Batteries[J]. Acta Chimica Sinica, 2013 , 71(03) : 427 -432 . DOI: 10.6023/A12110871
[1] Deng, C.; Shi, P.-F.; Zhang, S. Acta Chim. Sinica 2006, 64, 1031. (邓超, 史鹏飞, 张森, 化学学报, 2006, 64, 1031.)
[2] (a) Zhao, X. Y.; Ma, L. Q. Int. J. Hydrogen Energy 2009, 34, 4788;
(b) Liu, S.-Q.; Chen, D.-Y.; Huang, K.-L.; Zhong, X.-L. Acta Chim. Sinica 2009, 67, 513. (刘素琴, 陈东洋, 黄可龙, 仲晓铃, 化学学报, 2009, 67, 513.)
[3] Zhu, Y. F.; Zhang, W. F.; Yang, C.; Li, L. Q. Int. J. Hydrogen Energy 2010, 35, 9653.
[4] Yang, C. M.S. Thesis, Nanjing University of Technology, Nanjing, 2011. (杨尘, 硕士论文, 南京工业大学, 南京, 2011.)
[5] Mohamad, A. A.; Mohamed, N. S.; Alias, Y.; Arof, A. K. J. Alloys Compd. 2002, 337, 208.
[6] Yuan, A. B.; Zhao, J. Electrochim. Acta 2006, 51, 2454.
[7] Qiao, J.-L.; Fu, J.; Lin, R.; Ma, J.-X.; Liu, J.-S. Polymer 2010, 51, 4850.
[8] Yang, J.-M.; Chiang, C.-Y.; Wang, H.-Z.; Yang, C.-C. J. Membr. Sci. 2009, 341, 186.
[9] Mohamad, A. A.; Arof, A. K. Ionics 2008, 14, 415.
[10] (a) Yang, C.-C.; Chiu, S.-J.; Chien, W.-C.; Chiu, S.-S. J. Power Sources 2010, 195, 2212;
(b) Sang, S. B.; Wu, Q. M.; Gan, Z. Electrochim. Acta 2008, 53, 5065.
[11] Boudin, F.; Andrieu, X.; Jehoulet, C.; Olsen, I. I. J. Power Sources 1999, 81, 804.
[12] Yang, C. C.; Yang, J. M.; Wu, C. Y. J. Power Sources 2009, 191, 669.
[13] (a) Chen, Z.-Y.; Ju, Y.-L.; Zhang, W.-F.; Zhu, Y.-F.; Li, L.-Q. Mater. Rev. 2009, 23, 316. (陈忠元, 居亚兰, 张文峰, 朱云峰, 李李泉, 材料导报, 2009, 23, 316.);
(b) Chen, Z.-Y.; Ju, Y.-L.; Zhang, W.-F.; Zhu, Y.-F.; Li, L.-Q. J. Funct. Mater. 2008, 39, 527. (陈忠元, 居亚兰, 张文峰, 朱云峰, 李李泉, 功能材料, 2008, 39, 527.)
[14] Yang, C. C.; Chiu, S. S.; Kuo, S. C.; Liou, T. H. J. Power Sources 2011, 196, 4458.
[15] Rashkov, I.; Manolova, N.; Li, S. M.; Espartero, J. L.; Vert, M. Macromolecules 1996, 29, 50.
[16] (a) Li, Y.-J. M S. Thesis, Soochow University, Suzhou, 2011. (李延洁, 硕士论文, 苏州大学, 苏州, 2011);
(b) Yang, Y.-X.; Yang, B.-Y.; Duan, X.-J. Silicone Fluorine Info. 2004, 9, 27. (杨元秀, 杨本意, 段先健, 有机硅氟资讯, 2004, 9, 27.);
(c) Yang, Y.-X.; Yang, B.-Y.; Duan, X.-J. Silicone Fluorine Info. 2005, 11, 40. (杨元秀, 杨本意, 段先健, 有机硅氟资讯, 2005, 11, 40.);
(d) Zhang, W.-H.; Fan, X.-D.; Tian, W.; Fan, W.-W.; Cheng, G.-W. Acta Chim. Sinica 2011, 69, 2047. (张卫红, 范晓东, 田威, 范伟伟, 程广文, 化学学报, 2011, 69, 2047.)
[17] (a) Lue, S. J.; Mahesh, K. P. O.; Wang, W. T.; Chen, J. Y.; Yang, C. C. J. Membr. Sci. 2011, 367, 256;
(b) Yang, C. C.; Li, Y. J.; Liou, T. H. Desalination 2011, 276, 366;
(c) Kim, D. S.; Park, H. B.; Rhim, J. W.; Lee, W. M. J. Membr. Sci. 2004, 240, 37.
[18] (a) Saunier, J.; Gorecki, W.; Alloin, F.; Sanchez, J. Y. J. Phys. Chem. B 2005, 109, 2487;
(b) Kataoka, H.; Saito, Y.; Quaitarone, E.; Mustarelli, P. J. Phys. Chem. B 2000, 104, 11460.
[19] Yang, C. C. Mater. Sci. Eng. B 2006, 131, 256.
[20] (a) Michot, T.; Nishimoto, A.; Watanabe, M. Electrochim. Acta 2000, 45, 1347;
(b) Chen, Z.-Y. M.S. Thesis, Nanjing University of Technology, Nanjing, 2009. (陈忠元, 硕士论文, 南京工业大学, 南京, 2009);
(c) Suzuki, S.; Kimishima, K.; Takita, K. JP 2005082651-A, 2005 [Chem. Abstr. 2005, 20050331].
[21] Mohamad, A. A. Ionics 2005, 11, 294.
[22] Zhu, Y. F.; Wang, Y. C.; Li, L. Q. Int. J. Hydrogen Energy 2008, 33, 2965.
/
| 〈 |
|
〉 |