

Synthesis and Characterization of New Cyanostilbene-Based Compound Exhibiting Reversible Mechanochromism
Received date: 2013-01-06
Online published: 2013-02-20
Supported by
Project supported by the National Key Basic Research Program of China (Nos. 2010CB635108, 2011CBA00700), International Sci & Tech Cooperation Program, China (2012DFA51210) and the National Natural Science Foundation of China (Nos. 51203138, 51273179).
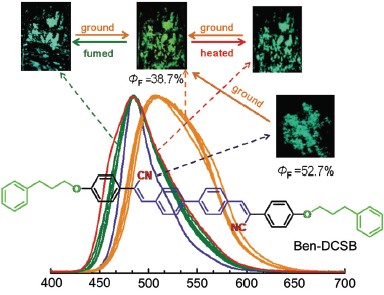
A rod-like molecules, 4,4'-bis(α-cyano-4-phenproalkoxylstryl)biphenyl (Ben-DCSB), has been synthesized and characterized by nuclear magnetic resonance (NMR), electron ionization-mass spectrometry (EI-MS), Fourier transform infrared (FR-IR) and elemental analysis. Ben-DCSB presents excellent mechanochromism behaviors. Recrystallized blue-green Ben-DCSB powder are changed into relatively yellow-green with the fluorescence quantum efficiency (ΦF) obviously converting from original 52.7% to 38.7% after being ground. Photoluminescence (PL) spectra of the samples obtained under different conditions (ungrind and grind) show the maximum emission wavelength (λem) has a significant red-shift as high as 23 nm after grinding. Observing the morphological structure of Ben-DCSB under different aggregative state by using scanning electron microscopy (SEM), the results indicate that the damage on surface morphology which is caused by external stimuli can be restored by exposing ethanol vapor or heat treatment. The X-ray diffractometry (XRD) measurements, the ungrind dye displays indicative of well-defined microcrystalline-like structures, and grinding dye is amorphous feature in this state. These observations further indicate the mechanochromism originates from the altering mode of molecular packing from the high-order to disorder by grinding. Time-resolved fluorescence spectrofluorometer analysis of ungrind ande grind dye show the attenuation exponential increases from single exponential of ungrinding to double-exponential of grinding. The result further demonstrate order degree of the grind sample decreases. The ground powder return to original colour by fuming with solvent (ethanol, dichloromethane, tetrahydrofuran or acetone) or heating at about 100 ℃ for 2 min. And the PL spectra show these samples can return original λem (485 nm). Moreover, dye Ben-DCSB indicates good reversibility of fluorescence conversion upon grinding-fuming and grinding-heating processes. XRD measurements indicate that the amorphous sample obtained by grinding can revert to crystalline state by fuming with ethanol and heating. Furthermore, the thermal analysis results show Ben-DCSB exists liquid crystalline phase of nematic (schlieren texture) between 194 ℃ and 212 ℃, and thermal decomposition temperature of Ben-DCSB is 362 ℃, indicating that the compound has better thermal stability.
Key words: mechanochromism; reversible; fluorescent material; molecular packing; cyanostilbene
Mao Wengang , Chen Kang , Ouyang Mi , Sun Jingwei , Zhou Yongbin , Song Qingbao , Zhang Cheng . Synthesis and Characterization of New Cyanostilbene-Based Compound Exhibiting Reversible Mechanochromism[J]. Acta Chimica Sinica, 2013 , 71(04) : 613 -618 . DOI: 10.6023/A13010025
/
| 〈 |
|
〉 |