

Theoretical Studies on the Single Water Molecule Effects on the Reaction of HOBr with OH·
Received date: 2013-01-12
Online published: 2013-03-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 41165007) and Science and Technology Foundation of GuiZhou Province, China (Nos. [2011]2107, [2012]2189), and Open Research Fund of Key Laboratory of Atmospheric Composition and Optical Radiation, Chinese Academy of Sciences (No. JJ1107) and Innovation Foundation for Graduate Students of Guizhou University.
In this article, the reactions of OH· with HOBr in the absence and presence of water are investigated using the CCSD(T) and B3LYP theoretical methods at the aug-cc-pVTZ basis set. The goal of the present investigation is to determine how the single water molecule can affect the reaction mechanisms and kinetics of OH·+HOBr and estimate the importance of water effects on the OH·+HOBr reaction. The calculated results show that there are two reaction channels and the corresponding pre-complexes for the reaction of OH·+HOBr. The barriers of the reaction OH·+HOBr are 1.13 kcal·mol-1, 2.02 kcal·mol-1, respectively, which are in good agreement with the previous experimental and theoretical results. In addition, the reaction of OH·+HOBr is very complex when one water molecule is introduced because there are six reaction pathways and corresponding pre-reactive complexes. In particular, the activated barriers of the reaction HOBr+H2O…HO· are about 1.00 kcal·mol-1 lower than those of the naked reaction OH·+HOBr. Additionally, to estimate the importance of these processes in the atmosphere, the rate constant is evaluated using the conventional transition state theory with Wigner tunneling correction. The calculated rate constant of the naked reaction is 1.77×10-13 cm3·molecule-1·s-1 at 298 K, which is consistent with the previous computational value. However, the rate constant of HOBr…H2O+OH· reaction is 50 times faster than that of the naked reaction OH·+HOBr. Combined with concentrations of these species in the atmosphere, the reaction of OH· with HOBr in the presence of water is less important than the naked reaction OH·+HOBr. However, water effects on the OH·+HOBr are very obvious, which is very similar to the reaction OH·+HOCl. Therefore, the present study provides further insight into water effects in the atmospheric chemistry.
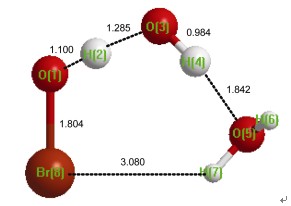
Key words: atmospheric chemistry; HOBr; OH·; water; reaction mechanism; reaction kinetics
Gao Chenggui , Long Zhengwen , Tan Xingfeng , Long Bo , Long Chaoyun , Qin Shuijie , Zhang Weijun . Theoretical Studies on the Single Water Molecule Effects on the Reaction of HOBr with OH·[J]. Acta Chimica Sinica, 2013 , 71(05) : 849 -856 . DOI: 10.6023/A13010058
/
| 〈 |
|
〉 |