

Regio-selective Dehydrogenation on the D or E Rings of Oleanolic Acid by Pd-Promoted C—H Activation
Received date: 2013-02-26
Online published: 2013-03-15
Supported by
Project supported by the National Basic Research Program of China (No. 2010CB529706).
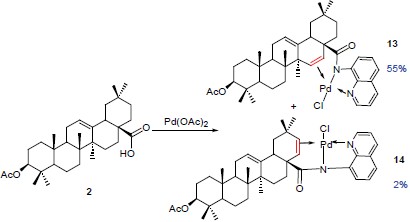
Oleanane-type triterpenes and their glycosides are a structurally and biologically diverse class of metabolites that are widely distributed in terrestrial plants and some marine organisms. Many of these compounds bear functional groups and modifications on the D and/or E rings of the triterpenes. These triterpenes compounds are of growing interest for drug research as they are active constituents of folk medicines and provide valuable pharmacological profiles. For their limited availability and accessibility, chemical synthesis provides a realistic way to determine the availability of homogenous natural products and their derivatives. Here, we report the selective dehydrogenation on the D or E rings of oleanoic acid by palladium promoted C—H activation with 8-aminoquinoline amide (substrate 12) and 2-aminomethylpyridine amide (substrate 16) as the directing groups. Thus, upon treatment with thionyl chloride, the 28-COOH of oleanoic acid was converted into 28-COCl, which coupled with the corresponding amines to provide the substrates readily for dehydrogenation. Notably, treatment of 12 with optimized conditions (1.0 equiv. Pd(OAc)2, 1.5 equiv. Oxone®, 1,2-dichloroethane, 80 ℃, 24 h) led to the dehydrogenated products 13 [double bond at C(15)—C(16)] and 14 [double bond at C(20)—C(21)] in 55% and 2% yields respectively. Treatment of 16 with optimized conditions (1.0 equiv. Pd(OAc)2, 2.0 equiv. Oxone®, 1,2-dichloroethane, microwave 85 ℃, 50 min) provided 17 [double bond at C(20)—C(21)] and 18 [double bond at C(15)—C(16)] in 42% and 20% yields respectively. Moreover, the distribution of the products varied in different solvents. It is worth mentioning that the N,N-bidentate ligands (two nitrogen atoms as the coordination sites in 12 and 16) are crucial for the palladium promoted olefination.
Ma Yuyong , Li Wei , Yu Biao . Regio-selective Dehydrogenation on the D or E Rings of Oleanolic Acid by Pd-Promoted C—H Activation[J]. Acta Chimica Sinica, 2013 , 71(04) : 541 -548 . DOI: 10.6023/A13020215
/
| 〈 |
|
〉 |