

Reaction Mechanism of Hydrogen Generation from Hydrolysis of BH4-: Quantum Chemistry Computing
Received date: 2013-01-15
Online published: 2013-03-21
Supported by
Project support by the National Basic Research Program of China (973 Program, No. 2009CB219906), the National Natural Science Foundation of China (No. 21276203) and Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20110201130002).
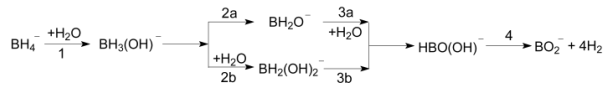
Borohydrides are considered to be one of the most promising hydrogen storage materials due to their high theoretical hydrogen storage gravimetric capacity. The recognition of reaction mechanism that the borohydrides generate hydrogen by hydrolysis can provide effective help for seeking the optimal reaction conditions. In this study, the mechanism for hydrogen evolution reaction between BH4- and two molecules of water has been investigated in detail by using the density functional theory (DFT) and the second-order Møller-Plesset perturbation theory (MP2). At the B3PW91/6-311++G(2df, 2p) and MP2(full)/6-311++G(2df, 2p) level of theory, the geometries of all species in the reaction system (reactants, isomers, transition states and products) were optimized. The transition states were validated by the vibrational frequency analysis and the calculations of the internal reaction coordinate (IRC). The computational results show that hydrogen is formed by a hydridic B—H hydrogen and a protic O—H hydrogen and that the reaction includes four steps in two possible reaction paths. In the first path, the water participates with the reaction in the first step and the third step, respectively; in the second path, the water participates with the reaction in the first step and the second step, respectively. Meanwhile, since the reaction barrier of each step is very high, the reaction is not easy to proceed spontaneously. The reaction barrier of the first step reaction is 252.0 kJ·mol-1 (in MP2 method) which is the highest in full steps, so that the first step is the control step of the reaction. In addition, both reaction paths are strong exothermic reaction process. The enthalpy change values obtained in MP2 method (-200.3 kJ·mol-1) is closer to the experimental observations (-216.7 kJ·mol-1). And during the reaction process, the hybridization of the center atom boron changes from sp3 hybrid to sp2 hybrid and then to sp hybrid.
Zhang Ying , Li Mingtao , Yang Bolun . Reaction Mechanism of Hydrogen Generation from Hydrolysis of BH4-: Quantum Chemistry Computing[J]. Acta Chimica Sinica, 2013 , 71(05) : 769 -776 . DOI: 10.6023/A13010070
/
| 〈 |
|
〉 |