

Study on 1-Octene Hydroformylation Promoted by Cetyltrihydroxyethyl Ammonium Bromide in Aqueous/Organic Biphasic Solution
Received date: 2013-02-01
Online published: 2013-03-21
Supported by
Project supported by Sichuan University Scientific Research Foundation for Young Teachers (No. 2011SCU11084) and Petrochemical Research Institute of China National Petroleum Corporation (No. 2011B-2606).
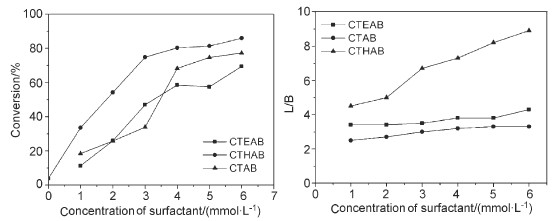
A persisting problem in homogenous olefin hydroformylation is the recovery and reuse of the expensive rhodium catalyst. The aqueous/organic biphasic catalysis offers a feasible way to solve the problem of separation of products from catalysts. However, owing to very low solubility of higher olefin in the aqueous phase and the higher energy barrier of olefin transfer from the organic phase to the aqueous phase, the reaction rate is extremely low. The addition of a surfactant is a practical way to accelerate long-chain alkene hydroformylation in aqueous/organic bisphasic solution. In this paper, the influence of cetyltrihydroxyethyl ammonium bromide (CTHAB) on 1-octene hydroformylation in aqueous/organic two-phase solution with HRh(CO)(TPPTS)3 as catalyst and TPPTS [P(m-C6H4SO3Na)3] as ligand was investigated. It was confirmed that a complex formed between CTHAB and HRh(CO)(TPPTS)2 and the complex was further led to the enrichment of the catalytically active species on the micelle surface. Compared with the traditional surfactant cetyltrimethyl ammonium bromide (CTAB), results showed that the addition of surfactant CTHAB not only accelerated the 1-octene hydroformylation but also improved the molar ratio of linear to branched aldehydes. The effects of various reaction parameters, such as concentration of surfactants, [TPPTS]/[Rh] molar ratio, pressure of syngas and recycling number of catalytic system were investigated. Under the mild conditions: [Rh]=0.8 mmol/L, [TPPTS]/[Rh]=40, [CTHAB]=4.0 mmol/L, 90 ℃, 0.5 MPa, 1.5 h, the TOF and the molar ratio of linear to branched aldehydes reached up to 497 h-1 and 25.6, respectively. The catalyst-containing aqueous could be easily separated from organic phase and efficiently reused for seven times without evident loss of activity and regioselectivity. Meanwhile, the hydroformylation of different chain length olefins was also promoted by CTHAB.
Su Ke , Jiang Hongbin , Zhu Deming , Fu Haiyan , Zheng Xueli , Yuan Maolin , Li Ruixiang , Chen Hua . Study on 1-Octene Hydroformylation Promoted by Cetyltrihydroxyethyl Ammonium Bromide in Aqueous/Organic Biphasic Solution[J]. Acta Chimica Sinica, 2013 , 71(05) : 844 -848 . DOI: 10.6023/A13020164
/
| 〈 |
|
〉 |