

Bisimidazole and Bisimidazolium Cruciforms: Synthesis and Discrimination of Organic Acids
Received date: 2013-04-01
Online published: 2013-06-27
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20902063, 21172159) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (No. 20111568-8-2).
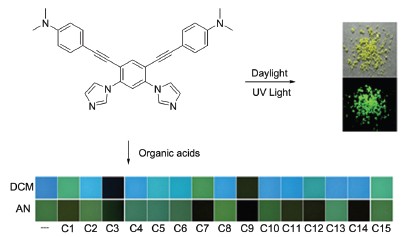
Bisimidazole cruciforms 4a&b based on 1,3-di(imidazol-1-yl)-benzene were synthesized in good yields via the Sonogashira coupling of 1,5-dibromo-2,4-diiodobenzene with 4-ethynyl-N,N-dimethylaniline and 4-ethynyl-N,N-dibutyl- aniline followed by the N-arylation with imidazole. Further quaternization of 4 with iodomethane afforded bisimidazolium cruciforms 5a&b. As an example, the didonor-π-diacceptor cruciform 4a showed strong solvatochromism that its emission colors varied from deep blue (toluene: 422 nm) to yellow (DMSO: 516 nm) in different organic solvents. The Stokes shift of 4a in acetonitrile was as large as 148 nm, which could suppress the fluorescence quench caused by reabsorption. The single bond connected multi-aromatic system made cruciform 4a possess aggregation-induced emission (AIE) property. A 12-fold enhancement of the fluorescence was observed for the solution of 4a in methanol/water (70:30, V/V) compared with in methanol/water (50:50, V/V). Taking advantage of the digital photography technology, fifteen carboxylic acids having either similar pKa values or similar structures were distinguished well by using bisimidazole cruciform 4a as the only indicator in two solvents. However, bisimidazolium cruciforms 5 could not discriminate them. All analytes were dissolved in dichloromethane and acetonitrile. A 20 μL of dilute stock solution of 4a was added into 2.0 mL of each such solution. The solutions were well-mixed and subjected to the UV illumination (365 nm). Photos were then taken using a digital camera one by one. The square segments representative of the emission colors were cut out and arranged into arrays for naked-eye discrimination. These emission colors were later converted into numerical R/G/B values by using Adobe PhotoShop. The standard deviations σs were calculated for the R/G/B value of the solution of 4a with each analyte towards those with the other analytes, which represented the degrees of discrimination between two analytes. The standard deviations σ's were calculated for the R/G/B values of the solutions of 4a with different analytes towards that of the blank solution of 4a, which represented the degrees of the color variation caused by the recognition. Comparing with traditional fluorescence sensing method, the present method is simple, efficient and time-saving. Bisimidazole cruciform 4a emitted solid-state florescence of green-yellow color with a quantum yield of 0.33 while bisimidazolium cruciform 5a emitted pure red color with a quantum yield of 0.14. They exhibited reversible color change under the UV irradiation when contacted with HCl and Et3N vapors, indicating they could serve as solid sensors for acid vapors. The fluorescence study also showed that the alkyl chains significantly affect the solid-state fluorescence of bisimidazolium cruciform 5.
Wang Zhi , Zhou Hongjun , Hu Junyi , You Jingsong , Gao Ge . Bisimidazole and Bisimidazolium Cruciforms: Synthesis and Discrimination of Organic Acids[J]. Acta Chimica Sinica, 2013 , 71(9) : 1257 -1264 . DOI: 10.6023/A13040352
/
| 〈 |
|
〉 |