

A Protocol for α-Bromination of β-Substituted Enones
Received date: 2013-06-01
Online published: 2013-08-14
Supported by
Project supported by National Basic Research Program of China (973 program, 2013CB836900), the National Natural Science Foundation of China (Nos. 21172235, 21222202, and 21290180), Shanghai Pujiang Plan (12PJ1410800), and Chinese Academy of Sciences.
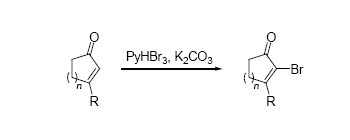
α-Haloenone is an important building block in organic synthesis, especially in natural product synthesis. There are at least 4 different reaction modes of this class of compounds: 1, crossing coupling reactions at the α-position; 2, conjugate additions at the β-position; 3, deprotonation at the α'-position; 4, deprotonation at the γ-position. Representative examples of the utilities of α-haloenone include the syntheses of diversonol and the core structures of lomaiviticins and guanacastepenes. From a mechanistic perspective, two types of α-halogenation methods have been developed previously: one through a sequential Baylis-Hillman-type Michael addition/α-halogenation/β-elimination process; the other one involving an electrophilic bromination followed by a β-elimination. The success of the former type of reactions highly depends on the formation of the transient enolate; the β-substituent on the enone substrates drastically slows down the rate of the first Michael addition step which is responsible for the enolate formation. The latter takes advantage of the strong electrophilicity of molecular bromine to overcome the steric effect of the substrates. However, quite a few side reactions including non-selective bromination often occur, resulting in modest to low yield and poor reproducibility of the desired product on a large scale. Here, we report a protocol for α-bromination of β-substituted enones using pyridine hydrobromide perbromide as a brominating reagent. Cyclic enones prove to be suitable substrates for this reaction, and the corresponding products were obtained in >80% yield in general. Notably, β-phenyl cyclohexenone, a rather unreactive substrate due to both steric and electronic reasons, was brominated smoothly. These bromination products may serve as useful synthon in organic synthesis. For acyclic enones and enoates, the reaction has to be performed at elevated temperature, which may be attributable to the rather poor acidity of the α-proton of the dibromo intermediate. The reaction is reliable on a large scale, and the reagents used are safe and low-toxic.
Yu Haixin , Wan Chunyun , Han Jing , Li Ang . A Protocol for α-Bromination of β-Substituted Enones[J]. Acta Chimica Sinica, 2013 , 71(11) : 1488 -1491 . DOI: 10.6023/A13060581
/
| 〈 |
|
〉 |