

Study on Synthesis, Rheological and Electrospinning Functional Materials of Carboxymethyl Cellulose Lithium (CMC-Li)
Received date: 2013-06-28
Online published: 2013-08-14
Supported by
Project supported by the the National Science-technology support plan projects (No. 2011BAD23B04), special programs of graduate students' scientific and technological innovative activities of Beijing institute of technology (No. 3090012241302) and Youth Science and Technology Innovation Projects of North Chemical Industry CO., LTD (No. QKCZ-BIT-04).
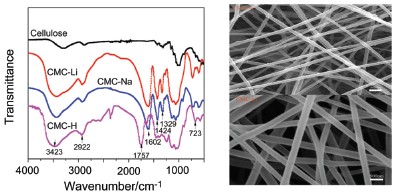
The study on electrospinning of natural polysaccharide polymer to be nano-crystallized is still difficult in the current time. Carboxymethyl cellulose sodium (CMC-Na) was successfully synthesized and acidized to obtain carboxymethyl cellulose hydrogen (CMC-H), and new ionic cellulose ether CMC-Li was synthesized by alkalization, and then we analyzed the difference of structure between the three products. By comparing the rheological measurement and analysis of CMC-Na and CMC-Li, which have different mass fractions, it is found that they both show the characteristics of non-Newtonian fluid. The non-Newtonian index of CMC-Li is lower than that of CMC-Na when the degree of substitution (DS) and viscosity of both is similar. For the same material, the ionic cellulose ether solution of lower DS and higher concentration shows the lower non-Newtonian index. For the structure and property characteristic of polymers, it was easy to combine them with water-soluble hydroxyl terminated high molecular weight polyethylene oxide (PEO) to allocate the excellent electrospinning solution. Processing conditions were adjusted to a flow rate of 4 mL/h, an applied voltage of 25 kV, the spinning distance of 12 cm, the better appearance of nanofibers whose average diameter is about 70 nm can be obtained. In the process of electrospinning, hydroxyl lithium ester enolates was obtained by reductive reaction of the ionized Li+ with active hydroxyl, which caused the molecular structure to be destroyed. CMC-Li showed the exceptional Non-Newtonian fluid properties and had the better spinning performance than CMC-Na. When the surface tension of liquid was damaged by external electric force of spinning voltage to balance each other, the obtained morphology of the fiber was better. Nano-aluminum powder is coated with CMC-Li to form composite nanofibers, and carbonized at the high temperatures. Al/CNF/Li composite fiber was obtained in which nano particles were uniformly distributed. We preliminarily studied the obtained composite material that remained morphology and structure of fiber, which lays the foundation for the later research that the material is applied to soluble membrane, tissue engineering scaffolds, biological medicine, lithium battery and super capacitor, etc. The satisfactory effects have been achieved through analysis of IR, Rheology and SEM. The paper expands the type of polysaccharide polymer that can be electrospun and filled the void of related research, and it also lays the foundation for the study on nano functional materials of new ionic cellulose ether.
Qiu Lei , Shao Ziqiang , Wang Jianquan , Zhang Dalun , Cao Jun . Study on Synthesis, Rheological and Electrospinning Functional Materials of Carboxymethyl Cellulose Lithium (CMC-Li)[J]. Acta Chimica Sinica, 2013 , 71(11) : 1521 -1526 . DOI: 10.6023/A13060680
[1] Liu, H.; Hsieh, Y. L. J. Polym. Sci., Part B: Polym. Phys. 2002, 40, 2119.
[2] Frey, M.; Joo, Y.; Kim, C. W. Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 2003, 44, 168.
[3] Zhao, S.; Wu, X.; Wang, L.; Huang, Y. Cellulose 2003, 10, 405.
[4] Yu, D. G.; Wang, X.; Li, X. Y.; Chian, W.; Li, Y.; Liao, Y. Z. Acta Biomater. 2013, 9, 5665.
[5] Shi, Y.; Zhang, J.; Xu, S.; Dong, A. J. J. Biomater. Sci., Polym. Ed. 2013, 24, 551.
[6] Xu, Y.-Z.; Dong, X.-T.; Gai, G.-Q.; Wang, J.-X.; Liu, G.-X.; Lu, T.-X. Acta Chim. Sinica 2012, 70, 1660. (徐淑芝, 董相廷, 盖广清, 王进贤, 刘桂霞, 鲁统晓, 化学学报, 2012, 70, 1660.)
[7] Li, H.; Shen, L.; Zhang, X.; Nie, P.; Chen, L.; Xu, K. J. Electrochem. Soc. 2012, 159, A426.
[8] Kong, J.; Liu, Z.; Yang, Z.; Tan, H. R.; Xiong, S.; Wong, S. Y.; Li, X.; Lu, X. Nanoscale 2012, 4, 525.
[9] Hwang, T. H.; Lee, Y. M.; Kong, B.-S.; Seo, J.-S.; Choi, J. W. Nano Lett. 2012, 12, 802.
[10] Zhang, J.; Liu, Z.; Kong, Q.; Zhang, C.; Pang, S.; Yue, L.; Wang, X.; Yao, J.; Cui, G. ACS Appl. Mater. Interfaces 2013, 5, 128.
[11] Li, Y.; Jiang, Y.; Liu, F.; Ren, F.; Zhao, G.; Leng, X. Food Hydrocolloid 2011, 25, 1098.
[12] Luo, Y.; Wang, S.; Shen, M.; Qi, R.; Fang, Y.; Guo, R.; Cai, H.; Cao, X.; Tomas, H.; Zhu, M.; Shi, X. Carbohydr. Polym. 2013, 91, 419.
[13] Nhu-Ngoc, B.; McCutcheon, J. R. Environ. Sci. Technol. 2013, 47, 1761.
[14] Manzine Costa, L. M.; de Olyveira, G. M.; Cherian, B. M.; Leao, A. L.; de Souza, S. F.; Ferreira, M. Ind. Crop. Prod. 2013, 41, 198.
[15] Nagy, Z. K.; Balogh, A.; Dravavoelgyi, G.; Ferguson, J.; Pataki, H.; Vajna, B.; Marosi, G. J. Pharm. Sci. 2013, 102, 508.
[16] Olaru, N.; Olaru, L.; Tudorachi, N.; Dunca, S.; Pintilie, M. Ind. Eng. Chem. Res. 2013, 52, 696.
[17] Ongun, M. Z.; Ertekin, K.; Hizliates, C. G.; Oter, O.; Ergun, Y.; Celik, E. Sens. Actuators B-Chem. 2013, 181, 244.
[18] Webster, M.; Miao, J.; Lynch, B.; Green, D. S.; Jones-Sawyer, R.; Linhardt, R. J.; Mendenhall, J. Macromol. Mater. Eng. 2013, 298, 447.
[19] Qiu, L.; Shao, Z.; Yang, M.; Wang, W.; Wang, F.; Xie, L.; Lv, S.; Zhang, Y. Carbohydr. Polym. 2013, 96, 240.
[20] Kang, Y. J.; Chun, S.-J.; Lee, S.-S.; Kim, B.-Y.; Kim, J. H.; Chung, H.; Lee, S.-Y.; Kim, W. ACS Nano 2012, 6, 6400.
[21] Lalia, B. S.; Samad, Y. A.; Hashaikeh, R. J. Solid State Electrochem. 2013, 17, 575.
[22] YerriSwamy, B.; Prasad, C. V.; Reedy, C. L. N.; Mallikarjuna, B.; Rao, K. C.; Subha, M. C. S. Cellulose 2011, 18, 349.
[23] Huang, X.-W.; Deng, J.-Y.; Xu, L.; Shen, P.; Zhao, B.; Tan, S.-T. Acta Chim. Sinica 2012, 70, 1604. (黄先威, 邓继勇, 许律, 沈平, 赵斌, 谭松庭, 化学学报, 2012, 70, 1604.)
[24] Prasanth, R.; Aravindan, V.; Srinivasan, M. J. Power Sources 2012, 202, 299.
[25] Wang, Y.-H.; Wang, J.-X.; Dong, X.-T.; Yu, W.-S.; Liu, G.-X. Acta Chim. Sinica 2012, 70, 1576. (王莹熇, 王进贤, 董相廷, 于文生, 刘桂霞, 化学学报, 2012, 70, 1576.)
[26] Awal, A.; Sain, M. J. Polym. Environ. 2012, 20, 690.
[27] Gao, Y. F.; Yuan, J. Y.; Sui, X. F.; Zhou, M.; Cai, Z. N. Prog. Chem. 2009, 21, 1553.
[28] Frey, M. W. Polym. Rev. 2008, 48, 378.
[29] Cui, M.; Wang, F.-J.; Shao, Z.-Q.; Lu, F.-S.; Wang, W.-J. Cellulose 2011, 18, 1265.
[30] Yang, Z. Y.; Wang, W. J.; Shao, Z. Q.; Zhu, H. D.; Li, Y. H.; Wang, F. J. Cellulose 2013, 20, 159.
[31] Konwarh, R.; Karak, N.; Misra, M. Biotechnol. Adv. 2013, 31, 421.
[32] Chang, C.; Zhang, L. Carbohydr. Polym. 2011, 84, 40.
[33] Kacmaz, S.; Ertekin, K.; Gocmenturk, M.; Suslu, A.; Ergun, Y.; Celik, E. React. Funct. Polym. 2013, 73, 674.
[34] Ma, G.; Shao, Z. Q.; Wang, W. J.; Wang, F. J.; Tan, L. L.; Liao, B. Sci. China. Chem. 2010, 53, 190.
[35] Xie, L.; Shao, Z.; Wang, W.; Wang, F. Integr. Ferroelectr. 2011, 127, 184.
[36] Lu, S.-Y.; Shao, Z.-Q.; Zhang, Z.-L.; Wang, H.-Q. Acta Chim. Sinica 2012, 70, 200. (吕少一, 邵自强, 张振玲, 王慧庆, 王文俊, 化学学报, 2012, 70, 200.)
[37] Qiu, L.; Shao, Z.; Yang, M.; Wang, W.; Wang, F.; Li, Y.; Chen, J. Adv. Mater. Res. 2013, 634-638, 2613.
[38] Zhao, T.; Liu, L.; Li, G.; Du, L.; Zhao, X.; Yan, J.; Cheng, Y.; Dang, A.; Li, T. Chin. Sci. Bull. 2012, 57, 1620.
[39] Wang, Z.; Dupre, N.; Gaillot, A.-C.; Lestriez, B.; Martin, J.-F.; Daniel, L.; Patoux, S.; Guyomard, D. Electrochim. Acta 2012, 62, 77.
[40] Reneker, D. H.; Yarin, A. L. Polymer 2008, 49, 2387.
[41] Wang, W.-K.; Zhang, Y.; Wang, A.-B.; Yu, Z.-B.; Han, M.-F.; Yang, Y.-S. Acta Phys.-Chim. Sin. 2010, 26, 47. (王维坤, 张勇, 王安邦, 余仲宝, 韩敏芳, 杨裕生, 物理化学学报, 2010, 26, 47.)
/
| 〈 |
|
〉 |