

Electronic Structures and Oxygen Ion Migration Energies of Metal Doped CeO2 Systems:A DFT+U Study
Received date: 2013-07-01
Online published: 2013-09-16
Supported by
Project supported by the Natural Science Foundation of Inner Mongolia (No. 2010BS0805) and "Chunhui plan" of the Ministry of Education (Z2009-1-01050).
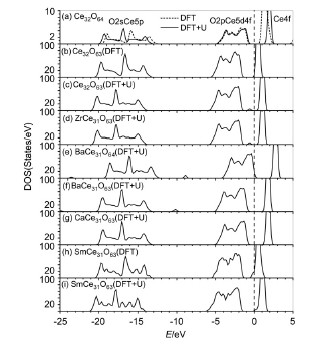
Doping energies of Ca, Ba, Sm and Zr in CeO2 systems and influences of dopings on oxygen ion migration energies and vacancy formation energies were studied using DFT and DFT+U methods. The calculated results showed that the doping energies increased with doping cation radius for doped CeO2 systems without oxygen vacancies, and for doped CeO2 systems with oxygen vacancies, the doping energies were related to the valence of the doping cations besides their radius. Calculations on electronic structures of various doped CeO2 systems showed that Fermi level shifted to the high energy in reduced CeO2, Zr-and Sm-doped CeO2 systems, however, in Ca-and Ba-doped CeO2 systems, negative charges due to the substitution of Ca2+ and Ba2+ with lower chemical valence for Ce4+ and positive charges due to the formation of oxygen ion vacancy were neutralized, so Fermi level scarcely shifted. Ce3+ existed in the reduced CeO2 system and the Zr-doped CeO2 system, which would lead to mixed conductivity with ion and electron one, and electron state of Ce3+ layed between Ce4f and O2p. However, the reduction of Ce4+ was restrained in Ca-, Ba-and Sm-doped CeO2 systems. The migration of an oxygen vacancy was investigated using the nudged elastic band method. The calculated results indicated that a straightforward migration path between two adjacent oxygen sites for oxygen vacancy hopping was obtained. For Ca-, Ba-, Sm-and Zr-doped CeO2 systems, the migration energies of oxygen ions were smaller than that of CeO2 system. In these doped CeO2 systems, the migration energy of oxygen ions for Ba was the smallest and its doping energy are the largest, so Ba was maybe introduced through adding the third class dopant in experiment.
Key words: cerium dioxide; DFT+U; doping energy; vacancy formation energy; ion migration energy
Jia Guixiao , Hao Wenxing , Pan Fei , Yang Jichun , Zhang Yongfan . Electronic Structures and Oxygen Ion Migration Energies of Metal Doped CeO2 Systems:A DFT+U Study[J]. Acta Chimica Sinica, 2013 , 71(12) : 1668 -1675 . DOI: 10.6023/A13070686
[1] Nam, K. W.; Kim, K. B. J. Electrochem. Soc. 2002, 149, A346.
[2] Song, X. W.; Zhao, Y. W.; Peng, J.; Zhao, W. G.; An, S. L. J. Funct. Mater. 2004, 35, 988. (宋希文, 赵永旺, 彭军, 赵文广, 安胜利, 功能材料, 2004, 35, 988.)
[3] Inaba, H.; Tagawa, H. Solid State Ionics 1996, 83, 1.
[4] Yoshida, H.; Deguchi, H.; Miura, K.; Horiuchi, M.; Inagaki, T. Solid State Ionics 2001, 140, 191.
[5] Yan, D. T.; Liu, X. M.; Bai, X. Y.; Pei, L.; Zheng, M. Z.; Zhu, C. J.; Wang, D. J.; Su, W. H. J. Power Sources 2010, 195, 6486.
[6] Huang, J. B.; Gao, Z.; Mao, Z. Q. Int. J. Hydrogen Energy 2010, 35, 4270.
[7] Kuharuangrong, S. J. Power Sources 2007, 171, 506.
[8] Yahiro, H.; Ohuchi, T.; Eguchi, K.; Arai, H. J. Mater. Sci. 1988, 23, 1036.
[9] Arai, H.; Kunisaki, T.; Shimizu, Y.; Seiyama, T. Solid State Ionics 1986, 20, 241.
[10] Pikalova, E. Y.; Maragou, V. I.; Demina, A. N.; Demin, A. K.; Tsiakaras, P. E. J. Power Sources 2008, 181, 199.
[11] Venkatasubramanian, A.; Gopalan, P.; Prasanna, T. R. S. Int. J. Hydrogen Energy 2010, 35, 4597.
[12] Andersson, D. A.; Simak, S. I.; Skorodumova, N. V.; Abrikosov, I. A.; Johansson, B. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 3518.
[13] Dudek, M.; Rapacz-Kmita, A.; Mroczkowska, M.; Mosia?ek, M.; Mordarski, G. Electrochim. Acta 2010, 55, 4387.
[14] van Herle, J.; Seneviratne, D.; McEvoy, A. J. J. Eur. Ceram. Soc. 1999, 19, 837.
[15] Nakayama, M.; Martin, M. Phys. Chem. Chem. Phys. 2009, 11, 3241.
[16] Wei, X.; Pan, W.; Cheng, L.; Li, B. Solid State Ionics 2009, 180, 13.
[17] Yoshida, H.; Inagaki, T.; Miura, K.; Inaba, M.; Ogumi, Z. Solid State Ionics 2003, 160, 109.
[18] Nitani, H.; Nakagawa, T.; Yamanouchi, M.; Osuki, T.; Yuya, M.; Yamamoto, T. A. Mater. Lett. 2004, 58, 2076.
[19] Frayret, C.; Villesuzanne, A.; Pouchard, M.; Mater, S. Int. J. Quantum Chem. 2005, 101, 826.
[20] Yang, Z. X.; Woo, T. K.; Hermansson, K. J. Chem. Phys. 2006, 124, 224704.
[21] Teng, B. T.; Jiang, S. Y.; Guo, X. W.; Yuan, J. H.; Luo, M. F. Acta Chim. Sinica 2009, 67, 2765. (滕波涛, 蒋仕宇, 郭晓伟, 袁金焕, 罗孟飞, 化学学报, 2009, 67, 2765.)
[22] Kresse, G.; Hafner, J. Phys. Rev. B 1994, 49, 14251.
[23] Kresse, G.; FurthmÜller, J. Comput. Mater. Sci. 1996, 6, 15.
[24] Perdew, J. P.; Burke, K.; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865.
[25] Feng, J.; Xiao, B.; Wan, C. L.; Qu, Z. X.; Huang, Z. C.; Chen, J. C.; Zhou, R.; Pan, W. Acta Mater. 2011, 59, 1742.
[26] Jónsson, H.; Mills, G.; Jacobsen, K. M. In Classical and Quantum Dynamics in Condensed Phase Simulations, Eds.: Berne, B. J.; Ciccotti, G.; Coker, D. F., World Scientific Publishing Co. Pte. Ltd., Singapore, 1998, pp. 385~404.
[27] Shi, S. Q.; Tang, Y. H.; Ouyang, C. Y.; Cui, L. X.; Xin, X. G.; Li, P. J.; Zhou, W. W.; Zhang, H.; Lei, M. S.; Chen, L. Q. J. Phys. Chem. Solids 2010, 71, 788.
[28] Liu, G.; Rodriguez, J. A.; Hrbek, J.; Dvorak, J.; Peden, C. H. F. J. Phys. Chem. B 2001, 105, 7762.
[29] Henderson, M. A.; Perkins, C. L.; Engelhard, M. H.; Thevuthasan, S.; Peden, C. H. F. Surf. Sci. 2003, 526, 1.
[30] Mullins, D. R.; Radulovic, P. V.; Overbury, S. H. Surf. Sci. 1999, 429, 186.
[31] Nolan, M.; Fearon, J. E.; Watson, G. W. Solid State Ionics 2006, 177, 3069.
[32] Steele, B. C. H.; Floyd, J. M. Proc. Br. Ceram. Soc. 1971, 19, 55.
/
| 〈 |
|
〉 |