

Design and Synthesis of a Metal-organic Framework with nia Topology
Received date: 2013-07-04
Online published: 2013-09-06
Supported by
Project supported by the National Natural Science Foundation of China (No. 20831002).
Nia topology is 3D structure which is constructed by octahedral and trigonal prism building blocks. In order to obtain Metal-organic Framework (MOFs) with nia topology, we can connect ligands with octahedral configuration and metal clusters with trigonal prism configuration. The typical metal clusters with trigonal prism configuration are M3O (M=metal, M is trivalent) or M3OH (M is divalent). In this article, a dendritic ligand 1,3,5-tris(3,5-di-(4-carboxy-phenyl-1-yl)phenyl-1-yl)benzene (TDCPB) with ten phenyl rings and an octahedral configuration is designed and synthesized. We used the metal salt of NiCl2 and the octahedral ligand of TDCPB to construct a MOF (JUC-105) with nia topology in solvent thermal condition. (Details: In a 20 mL reactor, NiCl2·6H2O (30 mg) and the dendritic ligand TDCPB (10 mg) are added to a solvent of Dimethylacetamide (DMA, 3 mL), and then 3 drops of HBF4 solution are added. After ultrasonic diffusion for 2 min, it is heated at 100 ℃ for 72 h to obtain green single crystals) Single crystal diffraction is used to characterize its structure. JUC-105 crystallizes in P-62c space group. JUC-105 is constructed by Ni3OH cluster and TDCPB with a 3D structure. The dimension of the large channel is about 1.1 nm. X-ray diffraction and thermogravimetric analysis are used to analyse its thermal stability. The gas uptake of JUC-105 is also tested. It is found that JUC-105 does not have N2 adsorption. It may be because the weak stability of the structure after the guest molecular removed. However, this MOF has a CO2 uptake of 24 mL/g at 273 K, 1 atm. After the collapsing of the structure, windows of the pores are blocked and the dimension of the pore is much smaller. Therefore, the N2 molecules can not be adsorbed. Because the CO2 have smaller dimension than N2 molecules, and it can pass through the small windows, the structure still have a comparative CO2 adsorption.
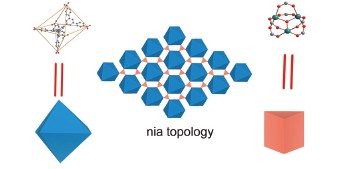
Key words: Metal-organic Framework (MOF); nia topology; CO2 adsorption; self-assembly
Jia Jiangtao , Wang Lei , Zhao Qing , Sun Fuxing , Zhu Guangshan . Design and Synthesis of a Metal-organic Framework with nia Topology[J]. Acta Chimica Sinica, 2013 , 71(11) : 1492 -1495 . DOI: 10.6023/A13070702
[1] (a) Chae, H. K.; Eddaoudi, M.; Kim, J.; Hauck, S. I.; Hartwig, J. F.; O'Keeffe, M.; Yaghi, O. M. J. Am. Chem. Soc. 2001, 123, 11482; (b) Reinsch, H.; Marszalek, B.; Wack, J.; Senker, J.; Gil, B.; Stock, N. Chem. Commun. 2012, 48, 9486.
[2] (a) Tanaka, K.; Muraoka, T.; Hirayama, D.; Ohnish, A. Chem. Commun. 2012, 48, 8577; (b) Zhang, X.-F.; An, X.-H.; Liu, D.-H.; Yang, Q.-Y.; Yang, Z.-H.; Zhong, C.-L.; Lu, X.-H. Acta Chim. Sinica 2011, 69, 84. (张秀芳, 安晓辉, 刘大欢, 阳庆元, 杨祝红, 仲崇立, 陆小华, 化学学报, 2011, 69, 84.); (c) Wu, X.-J.; Yang, X.; Song, J.; Cai, W.-Q. Acta Chim. Sinica 2012, 70, 2518. (吴选军, 杨旭, 宋杰, 蔡卫权, 化学学报, 2012, 70, 2518.) (d) Zhou, Z.-E.; Xue, C.-Y.; Yang, Q.-Y.; Zhong, C.-L. Acta Chim. Sinica 2009, 67, 477. (周子娥, 薛春瑜, 阳庆元, 仲崇立, 化学学报, 2009, 67, 477.)
[3] Song, F.; Wang, C.; Falkowski, J. M.; Ma, L.; Lin, W. J. Am. Chem. Soc. 2010, 132, 15390.
[4] Chen, B.; Wang, L.; Zapata, F.; Qian, G.; Lobkovsky, E. B. J. Am. Chem. Soc. 2008, 130, 6718.
[5] Taylor-Pashow, K. M.; Della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. J. Am. Chem. Soc. 2009, 131, 14261.
[6] Tranchemontagne, D. J.; Mendoza-Cortes, J. L.; O'Keeffe, M.; Yaghi, O. M. Chem. Soc. Rev. 2009, 38, 1257.
[7] Yaghi, O. M.; O'Keeffe, M.; Ockwig, N. W.; Chae, H. K.; Eddaoudi, M.; Kim, J. Nature 2003, 423, 705.
[8] Delgado-Friedrichs, O.; O'Keeffe, M.; Yaghi, O. M. Acta Crystallogr. A 2006, 62, 350.
[9] Wang, Z.; Zhang, X.; Batten, S. R.; Kurmoo, M.; Gao, S. Inorg. Chem. 2007, 46, 8439.
[10] Chae, H. K.; Eddaoudi, M.; Kim, J.; Hauck, S. I.; Hartwig, J. F.; O'Keeffe, M.; Yaghi, O. M. J. Am. Chem. Soc. 2001, 123, 11482.
[11] Bai, Y. L.; Tao, J.; Huang, R. B.; Zheng, L. S.; Zheng, S. L.; Oshida, K.; Einaga, Y. Chem. Commun. 2008, 1753.
[12] Jia, J.; Sun, F.; Fang, Q.; Liang, X.; Cai, K.; Bian, Z.; Zhao, H.; Gao, L.; Zhu, G. Chem. Commun. 2011, 47, 9167.
[13] Jia, J.; Sun, F.; Borjigin, T.; Ren, H.; Zhang, T.; Bian, Z.; Gao, L.; Zhu, G. Chem. Commun. 2012, 48, 6010.
[14] Jia, J.; Lin, X.; Wilson, C.; Blake, A. J.; Champness, N. R.; Hubberstey, P.; Walker, G.; Cussen, E. J.; Schroder, M. Chem. Commun. 2007, 840.
/
| 〈 |
|
〉 |