

Reflux Precipitation Polymerization:A New Technology for Preparation of Monodisperse Polymer Nanohydrogels
Received date: 2013-07-24
Online published: 2013-08-21
Supported by
Project supported by National Science and Technology Key Project of China (No. 2012AA020204), the National Natural Science Foundation of China (Nos. 21034003, 21128001 and 51073040).
Monodisperse polymer microspheres have been widely used in chromatography separation, coating additives, biological separation and targeted drug delivery due to their varying properties, such as the various morphology, well-defined composition, functional surface and biocompatibility. Recently, the preparation of monodisperse polymer microspheres and nanohydrogels has attracted great interests. Considering the shortage of the preparation method of the polymer nanohydrogels, we developed a new technique—reflux precipitation polymerization—for preparation of monodisperse and biocompatible polymer nanohydrogels. A typical procedure for the reflux precipitation polymerization is described as follows: Firstly, a suitable amount of monomers[MAA (methacrylic acid) and DVB (divinylbenzene)] and initiator[AIBN (2,2-azobisisobutyronitrile)] dissolved in acetonitrile was added into a round-bottom flask configured with a condenser. When the reaction temperature increased to the boiling point of the solution for about 5 min, the initially transparent reaction solution became milky white, and the reaction was continued for another 2 h. Then the resultant microspheres were separated and purified by repeating ultra-centrifugation (12000 r/min for 3 min)-decanting-redispersion (in ethanol with ultrasonic bathing) for three times. The morphology and particle size distribution of PMAA nanohydrogels were characterized by scanning electron microscope (SEM) and dynamic light scattering (DLS). Compared to the traditional precipitation polymerization and distillation-precipitation polymerization, this new method has many merits, such as high efficiency, good repeatability and easy operation, which can be used to high efficient preparation of polymer nanohydrogels and the related composite microspheres. Through testing the reaction conditions, such as reaction time, solid content, cross-linker content and solvent mixture, we successfully prepared the monodisperse PMAA nanohydrogels, and obtained the fundamental rule for the morphology control. We found that the diameter of PMAA nanohydrogels became larger and larger as the reaction time extended or the solid content and/or the ratio of good solvent of polymer increased. The particle size distribution of the PMAA nanohydrogels became poor if the solid content or the cross-linker content was beyond a certain threshold. These nanohydrogels and the related composite microspheres prepared by this new technique will be widely used in the biomedical fields.
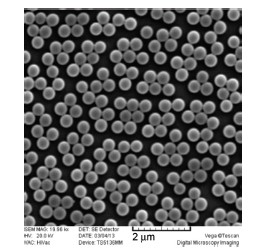
Jin Sha , Pan Yuanjia , Wang Changchun . Reflux Precipitation Polymerization:A New Technology for Preparation of Monodisperse Polymer Nanohydrogels[J]. Acta Chimica Sinica, 2013 , 71(11) : 1500 -1504 . DOI: 10.6023/A13070776
[1] Elsabahy, M.; Wooley, K. L. Chem. Soc. Rev. 2012, 41, 2545.
[2] McDonald, C. J.; Bouck, K. J.; Chaupt, A. B. Macromolecules 2000, 33, 1593.
[3] Poma, A.; Turner, A. P. F.; Piletsky, S. A. Trends Biotechnol. 2010, 28, 629.
[4] Li, W.-H.; Stöver, H. D. H.; Hamielec, A. E. J. Polym. Sci., Part A: Polym. Chem. 1994, 32, 2029.
[5] Ma, G.-H.; Fukutomi, T.; Nazaki, S. J. Appl. Polym. Sci. 1997, 63, 1243.
[6] Arshady, R. Polym. Eng. Sci. 1990, 30, 905.
[7] Meng, J.-Y.; Gao, B.-J.; Chen, Z.-P.; Yao, L. Acta Chim. Sinica 2012, 70, 2273. (门吉英, 高保娇, 陈志萍, 幺兰, 化学学报, 2012, 70, 2273.)
[8] Kawaguchi, H. Prog. Polym. Sci. 2000, 25, 1171.
[9] Hao, Z.-X.; Cheng, Y.-Y.; Wang, L.-L.; Wang, X.-G.; Zhu, Z.-R.; Gan, L.-H.; Xu, Z.-J.; Chen, L.-W. Acta Chim. Sinica 2012, 70, 331. (郝志显, 程艺艺, 王乐乐, 王晓岗, 朱志荣, 甘礼华, 徐子颉, 陈龙武, 化学学报, 2012, 70, 331.)
[10] Oh, J. K.; Drumright, R.; Siegwart, D. J.; Matyjaszewski, K. Prog. Polym. Sci. 2008, 33, 448.
[11] Li, K.; Stöver, H. D. H. J. Polym. Sci., Part A: Polym. Chem. 1993, 31, 3257.
[12] Wu, J.-Z.; Huang, G.; Hu, Z.-B. Macromolecules 2003, 36, 440.
[13] Zhang, Q.-S.; Tang, Y.-C.; Zha, L.-S.; Ma, J.-H.; Liang, B.-R. Eur. Polym. J. 2008, 44, 1358.
[14] Halperin, A.; Kroeger, M. Macromolecules 2011, 44, 6986.
[15] Qian, J.; Wu, F.-P. J. Mater. Chem. B 2013, 1, 3464.
[16] Bai, F.; Yang, X.-L.; Li, R.; Huang, B.; Huang, W.-Q. Polymer 2007, 47, 5775.
[17] Dai, Z.; Yang, X.-L.; Huang, W.-Q. Polym. Int. 2007, 56, 224.
[18] Bilalis, P.; Boukos, N.; Kordas, G. C. Mater. Lett. 2012, 67, 180.
[19] Ji, M.; Liu, B.; Yang, X.-L.; Wang, J.-Y. Polymer 2009, 50, 5970.
[20] Pan, Y.-J.; Li, D.; Jin, S.; Wei, C.; Wu, K.-Y.; Guo, J.; Wang, C.-C. Polym. Chem. 2013, 4, 3545.
[21] Ma, M.-F.; Wu, K.-Y.; Tang, J.; Li, D.; Wei, C.; Guo, J.; Wang, S.-L.; Wang, C.-C. J. Mater. Chem. 2012, 22, 15206.
[22] Ma, W.-F.; Zhang, Y.; Li, L.-L.; Zhang, Y.-T.; Yu, M.; Guo, J.; Lu, H.-J.; Wang, C.-C. Adv. Funct. Mater. 2013, 23, 107.
[23] Pan, Y.-J.; Chen, Y.-Y.; Wang, D.-R.; Wei, C.; Guo, J.; Lu, D.-R.; Chu, C.-C.; Wang, C.-C. Biomaterials 2012, 33, 6570.
[24] Bai, F.; Yang, X. L.; Huang, W.-Q. Macromolecules 2004, 37, 9746.
/
| 〈 |
|
〉 |