

Synthesis and Self-Assembled Structure of A Cluster-Cluster Hybrid Molecule Composed of POM and POSS Clusters
Received date: 2013-08-06
Online published: 2013-09-24
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21274069 and 21334003) and Open Research Fund of State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry.
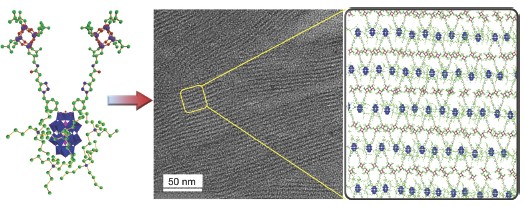
Polyhedral oligosilsesquioxane (POSS) and polyoxometalate (POM) are two kinds of clusters having totally different physical and chemical properties. For instance, the POSS cluster dissolves in weakly polar solvents, such as toluene, while the POM cluster, encapsulated by tetrabutylammonium counterions, dissolves in strongly polar solvents, such as acetonitrile, meaning the strong incompatibility. Based on this reason and their fixed shape, a novel cluster-cluster hybrid molecule with a V-shaped molecular structure (POM-2POSS) was rationally designed by covalently linking the two POSS clusters on the one side of the POM cluster. In the experiment, a two-azido-containing organosilyl derivative of a Wells-Dawson-type POM cluster and a one-propargyl-containing derivative of a POSS cluster were prepared at first. Then, the cluster-cluster hybrid was successfully synthesized by Cu-catalyzed click reaction between the two azide groups in the one POM derivative and the two propargyl groups in the two POSS derivatives. The chemical structure of this hybrid molecule was carefully characterized by NMR, ESI-MS and IR. In view of their strong incompatibility and of the particular three-dimensional (3D) structure POM-2POSS was expected to be able to self-assemble into ordered supramolecular structures. In the sample preparation POM-2POSS was dissolved in acetonitrile with a concentration of 5 mg/mL, and then the solution was dropped onto silicon substrates to prepare the thin film samples, finally the thin film samples were annealed in an acetonitrile vapor for 14 d. The film on the silicon substrate was characterized by XRD. The thin film samples for TEM characterization were made at first by floating onto the water surface and then transferred onto copper mesh. The structural analyses clearly demonstrated that the hybrid molecule self-assembled into a highly ordered lamellar morphology with a 5.1 nm periodicity, smaller than those found in block copolymers with a similar molecular weight. Formation of the highly ordered morphology reflects a self-assembly process due to absence of intermolecular entanglements, while the sub-5 nm periodicity is because of the 3D structures of the two building blocks. The findings provide a new platform for understanding of the self-assembly of nano-clusters and for development of novel hybrid materials.
Hou Zhanyao , Hu Minbiao , Wang Wei . Synthesis and Self-Assembled Structure of A Cluster-Cluster Hybrid Molecule Composed of POM and POSS Clusters[J]. Acta Chimica Sinica, 2014 , 72(1) : 61 -68 . DOI: 10.6023/A13080821
[1] Gómez-Romero, P.; Sanchez, C. Functional Hybrid Materials, Wiley-VCH, Weinheim, 2006.
[2] Kickelbick, G. Hybrid Materials: Synthesis, Characterization, and Applications, Wiley-VCH, Weinheim, 2007.
[3] Clément, S.; Kenneth, J. S.; Susumu, K. Chem. Soc. Rev. 2011, 40, 471.
[4] Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. J. Mater. Chem. 2005, 15, 3559.
[5] Zhang, Z. L.; Horsch, M. A.; Lamm, M. H.; Glotzer, S. C. Nano Lett. 2003, 3, 1341.
[6] Glotzer, S. C.; Horsch, M. A.; Iacovella, C. R.; Zhang, Z. L.; Chan, E. R.; Zhang, X. Curr. Opin. Colloid Interface Sci. 2005, 10, 287.
[7] Šari?, A.; Bozorgui, B.; Cacciuto, A. J. Phys. Chem. B 2011, 115, 7182.
[8] Filion, L.; Marechal, M.; Oorschot, B. V.; Pelt, D.; Smallenburg, F.; Dijkstra, M. Phys. Rev. Lett. 2009, 103, 188302.
[9] Pradeep, C. P.; Misdrahi, M. F.; Li, F. Y.; Zhang, J.; Xu, L.; Long, D. L.; Liu, T. B.; Cronin, L. Angew. Chem., Int. Ed. 2009, 48, 8309.
[10] Han, Y. K.; Xiao, Y.; Zhang, Z. J.; Liu, B.; Zheng, P.; He, S. J.; Wang, W. Macromolecules 2009, 42, 6543.
[11] Hu, M. B.; Xia, N.; Yu, W.; Ma, C.; Tang, J.; Hou, Z. Y.; Zheng, P.; Wang, W. Polym. Chem. 2012, 3, 617.
[12] Landsmann, S.; Lizandara-Pueyo, C.; Polarz, S. J. Am. Chem. Soc. 2010, 132, 5315.
[13] Landsmann, S.; Wessig, M.; Schmid, M.; Cölfen, H.; Polarz, S. Angew. Chem., Int. Ed. 2012, 51, 5995.
[14] Hu, M. B.; Hou, Z. Y.; Xiao, Y.; Yu, W.; Ma, C.; Ren, L. J.; Zheng, P.; Wang, W. Langmuir 2013, 29, 5714.
[15] Wang, X. L.; Wang, Y. L.; Miao, W. K.; Hu, M. B.; Tang, J.; Yu, W.; Hou, Z. Y.; Zheng, P.; Wang, W. Langmuir 2013, 29, 6537.
[16] Yu, X. F.; Zhong, S.; Li, X. P.; Tu, Y. F.; Yang, S. G.; Van Horn, R. M.; Ni, C. Y.; Pochan, D. J.; Quirk, R. P.; Wesdemiotis, C.; Zhang, W. B.; Cheng, S. Z. D. J. Am. Chem. Soc. 2010, 132, 16741.
[17] Li, Y.; Zhang, W. B.; Hsieh, I.; Zhang, G. L.; Cao, Y.; Li, X. P.; Wesdemiotis, C.; Lotz, B.; Xiong, H. M.; Cheng, S. Z. D. J. Am. Chem. Soc. 2011, 133, 10712.
[18] Sun, H. J.; Tu, Y. F.; Wang, C. L.; Van Horn, R. M.; Tsai, C. C.; Graham, M. J.; Sun, B.; Lotz, B.; Zhang, W. B.; Cheng, S. Z. D. J. Mater. Chem. 2011, 21, 14240.
[19] Yu, X. F.; Zhang, W. B.; Yue, K.; Li, X. P.; Liu, H.; Xin, Y.; Wang, C. L.; Wesdemiotis, C.; Cheng, S. Z. D. J. Am. Chem. Soc. 2012, 134, 7780.
[20] Polyoxometalate Chemistry: From Topology via Self-Assembly to Applications, Eds.: Pope, M. T.; Müller, A., Springer, Netherlands, 2001.
[21] Special thematic issue on polyoxometalates: Chem. Rev. 1998, 98, 1–388.
[22] Proust, A.; Thouvenot, R.; Gouzerh, P. Chem. Commun. 2008, 1837.
[23] Dolbecq, A.; Dumas, E.; Mayer, C. R.; Mialane, P. Chem. Rev. 2010, 110, 6009.
[24] Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Chem. Soc. Rev. 2012, 41, 7605.
[25] Marcella, B.; Mauro, C.; Gianfranco, S.; Alessandro, B. Adv. Synth. Catal. 2004, 346, 648.
[26] Cordes, D. B.; Lickiss, P. D.; Rataboul, F. Chem. Rev. 2010, 110, 2081.
[27] Tanaka, K.; Chujo, Y. J. Mater. Chem. 2012, 22, 1733.
[28] Odobel, F.; Severac, M.; Pellegrin, Y.; Blart, E.; Fosse, C.; Cannizzo, C.; Mayer, C. R.; Elliott, K. J.; Harriman, A. Chem. Eur. J. 2009, 15, 3130.
[29] Contant, R.; Klemperer, W. G.; Yaghi, O., In Inorganic Syntheses, Mc Graw-Hill, New York, 1990, Vol. 27, p. 104.
[30] Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004.
[31] Hamley, I. W. The Physics of Block Copolymers, Oxford University Press, Oxford, 1999.
/
| 〈 |
|
〉 |