

Aqueous Synthesis of Near-Infrared CdTe Quantum Dots for Biothiols Detection in Biological Fluids
Received date: 2013-09-05
Online published: 2013-11-14
Supported by
Project supported by the National Natural Science Foundation of China (No. 21205087).
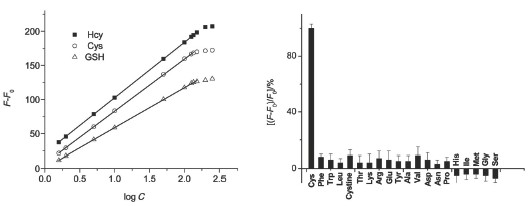
As well known that conventional aqueous synthesis of the near-infrared (NIR) CdTe quantum dots (QDs) using thiol ligands as capping reagents is usually very time-consuming. To overcome this defect and prepare NIR CdTe QDs, we present a fast and facile route in aqoueous phase under atmospheric pressure using L-cysteine (L-Cys) as capping reagents. The influences of various experimental conditions on the growth rate and luminescent properties of the obtained CdTe QDs have been systematically investigated, including Te-to-Cd ratio, L-Cys-to-Cd ratio and pH value. The experiment results suggested that lower ratio of Te:Cd or L-Cys:Cd and high pH value would markedly shortened the reaction time. Furthermore, the obtained QDs were used as a kind of NIR fluorescent probes for thiol detection in biological fluids. The change in the fluorescence intensity of the NIR CdTe QDs in the presence of 5.0 μmol·L-1 homocysteine (Hcy), L-Cys or glutathione (GSH) with different interaction time was measured. The effect of pH on the enhanced fluorescence intensity of the NIR CdTe QDs (10 mg·L-1) at 5.0 μmol·L-1 Hcy, L-Cys or GSH and the fluorescence responses of NIR CdTe QDs to 20 essential amino acids (5.0 mmol·L-1 for L-Cys, 5.0 mmol·L-1 for the other 19 amino acids) in pH 7.0 PBS buffer was investigated. The probe offered good sensitivity and selectivity for detecting L-Cys, Hcy and GSH in the presence of 20 other amino acids, main relevant metal ions, and some other molecules in biological fluids. The recovery of spiked 5.0 mmol·L-1 thiols in human serum and cell extracts ranged from 90% to 109%. The precision for 11 replicate measurements of the thiols at 5.0 mmol·L-1 is in the range of 2.4%~3.3%. The detection limits (3s) for L-Cys, Hcy and GSH are 43, 46 and 63 nmol·L-1, respectively. For real sample measurement, four serum samples and cell extract sample from two cancer cell lines (Hela and HepG2) were chosen and the analytical results were comparable with HPLC assay.
Cui Xiaoteng , Lv Yuyang , Liu Ying , Wu Boyue . Aqueous Synthesis of Near-Infrared CdTe Quantum Dots for Biothiols Detection in Biological Fluids[J]. Acta Chimica Sinica, 2014 , 72(1) : 75 -82 . DOI: 10.6023/A13090931
[1] Zhang, M.; Yin, B. C.; Tan, W. H.; Ye, B. C. Biosens. Bioelectron. 2011, 26, 3260.
[2] Zhang, M.; Ye, B. C. Anal. Chem. 2011, 83, 1504.
[3] Yoshitake, M.; Nohta, H.; Sejima, N.; Todoroki, K.; Yoshida, H.; Yamaguchi, M. Anal. Bioanal. Chem. 2011, 399, 1665.
[4] Tay, L. L.; Tremblay, R. G.; Hulse, J.; Zurakowski, B.; Thompson, M.; Bani-Yaghoub, M. Analyst 2011, 136, 1620.
[5] Sun, S. K.; Wang, H. F.; Yan, X. P. Chem. Commun. 2011, 47, 3817.
[6] Niu, H.; Yuan, R.; Chai, Y.; Mao, L.; Yuan, Y.; Zhuo, Y.; Yuan, S.; Yang, X. Biosens. Bioelectron. 2011, 26, 3175.
[7] Yang, T.; Chen, M. L.; Hu, X. W.; Wang, Z. W.; Wang, J. H.; Dasgupta, P. K. Analyst 2011, 136, 83.
[8] Shao, J.; Guo, H.; Ji, S.; Zhao, J. Biosens. Bioelectron. 2011, 26, 3012.
[9] Zuo, Q. P.; Li, B.; Pei, Q.; Li, Z. J.; Liu, S. K. J. Fluoresc. 2010, 20, 1307.
[10] Huang, S.-T.; Ting, K.-N.; Wang, K.-L. Anal. Chim. Acta 2008, 620, 120.
[11] Wang, J. G.; Lv, H.; Sun, Q. H.; Li, R. Z.; Zhong, Y.; Zhao, J.; Liu, M.; Jia, X. Acta Chim. Sinica 2009, 67, 415.(王建国, 吕慧, 孙巧花, 李荣志, 钟岩, 赵健, 刘敏, 贾香, 化学学报,2009, 67, 415.)
[12] Xiong, L. Q.; Zhao, Q.; Chen, H. L.; Wu, Y. B.; Dong, Z. S.; Zhou, Z. G.; Li, F. Y. Inorg. Chem. 2010, 49, 6402.
[13] Lim, S.; Escobedo, J. O.; Lowry, M.; Xu, X. Y.; Strongin, R. Chem. Commun. 2010, 46, 5707.
[14] Park, K. S.; Kim, M. II; Woo, M.-A.; Park, H. G. Biosens. Bioelectron. 2013, 45, 65.
[15] Zechmann, B.; Tomasic, A.; Horvat, L.; Fulgosi, H. Protoplasma 2010, 246, 65.
[16] Wang, H.; Xian, M. Curr. Opin. Chem. Biol. 2011, 15, 32.
[17] Han, B.; Wang, E. Biosens. Bioelectron. 2011, 26, 2585.
[18] Dong, Y.; Li, J. Org. Lett. 2011, 13, 2252.
[19] Yuan, L. Org. Lett. 2011, 14, 432.
[20] Liu, T. T.; Peng, C.; Ma, Y. C.; Ouyang, J. Acta Chim. Sinica 2013, 71, 962. (刘亭廷, 彭程, 马云川, 欧阳津, 化学学报, 2013, 71, 962).
[21] Xie, W. J.; Fu, Y. Y.; Ma, H.; Zhang, M.; Fan, L. Z. Acta Chim. Sinica 2012, 70, 2169. (谢文菁, 傅英懿, 马红, 张沫, 范楼珍, 化学学报, 2012, 70, 2169).
[22] Yang, F.; Wang, L. L.; Guo, Z. H. Acta Chim. Sinica 2012, 70, 1283. (杨帆, 王伶俐, 郭志慧, 化学学报, 2012, 70, 1283).
[23] Liu, Z. Q.; Liu, S. P.; Yan, S. G.; Yin, P. F.; He, R. Q. Acta Chim. Sinica 2011, 69, 2969. (刘正清, 刘绍璞, 闫曙光, 殷鹏飞, 何佑秋, 化学学报, 2011, 69, 2969).
[24] Xu, J. P.; Jia, L.; Fang, Y. A.; Lv, L. P.; Song, Z. G.; Ji, J. A. Analyst 2010, 135, 2323.
[25] Thomas, J.; Sherman, D. B.; Amiss, T. J.; Andaluz, S. A.; Pitner, J. B. Bioconjugate Chem. 2007, 18, 1841.
[26] Yuan, J.; Guo, W.; Wang, E. Anal. Chem. 2008, 80, 1141.
[27] Ali, E. M.; Zheng, Y.; Yu, H.-H.; Ying, J. Y. Anal. Chem. 2007, 79, 9452.
[28] Suzuki, M.; Husimi, Y.; Komatsu, H.; Suzuki, K.; Douglas, K. T. J. Am. Chem. Soc. 2008, 130, 5720.
[29] He, Y.; Wang, H.-F.; Yan, X.-P. Anal. Chem. 2008, 80, 3832.
[30] Zou, L.; Gu, Z.; Zhang, N.; Zhang, Y.; Fang, Z.; Zhu, W.; Zhong, X. J. Mater. Chem. 2008, 18, 2807.
[31] Zhang, Y.; Li, Y.; Yan, X.-P. Anal. Chem. 2009, 81, 5001.
/
| 〈 |
|
〉 |