

Post-target Analysis for the Volatile Compounds from Salty Alpinia oxyphyllae Fructus with Headspace-gas Chromatography-quadrupole/time of Flight Mass Spectrometry
Received date: 2013-09-24
Online published: 2013-12-17
Supported by
Project supported by Shanghai Doctoral Degree Construction Project, the Innovation Program of Shanghai Municipal Education Commission (No.12ZZ121), Innovation Method Fund of China (No. 2011IM030200) and the National Natural Science Foundation of China (No. 21275155).
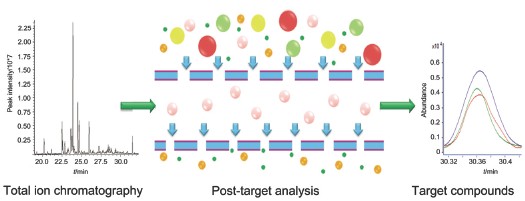
Post-target analysis is a superior method for qualitative analysis. This method can make a rapid, accurate and wide-scope screening for non-target and target compounds, and it has been used widely in the metabonomics, environmental analysis and pesticide residues. With post-target method to analysis non-target compounds, retention index, accurate mass and library were applied to investigate non-target analytes and then a total of 119 compounds were identified. Within those 119 compounds, there are different kinds of compounds, such as aliphatic aldehyde, aromatic ketone, aromatic aldehyde, monoterpene and its oxide, sesquiterpenes and its oxide and so on. Eremophilene is the best peak ingredient, p-cymene, aromadendrene, nootkatone and δ-cadinene are the main ingredients. Besides, nootkatone is the main active ingredient according to previous pharmacology research. Narrow mass window extracted ion chromatograms (nw-XIC) can decrease background noise greatly and improve the signal-to-noise rate. Hence, the trace level or co-elution compounds can be identified. With the post-target analytical method for target analytes, we calculated retention time from retention index of target compounds, and then nw-XIC (mass window 0.02 Da) was carried out. Four characteristic ions of target compounds were extracted to get extracted ion chromatograms at calculated retention time. As peaks at calculated retention time had the same chromatographic behavior, we identified three target compounds: nootkatol, α-cyperone and perillaldehyde. Tandem mass technology has been widely used since its introduction in the 1970s, it was used in trace analysis, chemical reaction mechanistic studies and propose the fragmentation pattern of compounds. In this paper, we got first-stage mass spectra and second-stage mass spectra data in one experiment. Moreover, we could obtain accurate mass of precursor ion and product ion from second-stage mass spectra at the same time, then we inferred the MS fragmentation pattern of compounds. This method is more simple, convenient and accurate than the traditional tandem mass spectrometry. Myrtenal was identified in this post-target way. Post-target analytical method can distinguish compounds from complex matrix samples as many as possible. This method solves the problems of co-elution and high background and can be used to investigate the medicine's pharmacology and toxicology mechanism.
Chen Fangjiao , Su Yue , Guo Yinlong . Post-target Analysis for the Volatile Compounds from Salty Alpinia oxyphyllae Fructus with Headspace-gas Chromatography-quadrupole/time of Flight Mass Spectrometry[J]. Acta Chimica Sinica, 2014 , 72(1) : 95 -104 . DOI: 10.6023/A13091003
[1] Hernandez, F.; Portoles, T.; Pitarch, E.; Lopez, F. J. Anal. Chem. 2007, 79, 9494.
[2] Zhang, F.; Yu, C.-T.; Wang, W.-W.; Fan, R.-J.; Zhang, Z.-X.; Guo, Y.-L. Anal. Chim. Acta 2012, 757, 39.
[3] Kang, W.-Y.; Zhang, F.; Su, Y.; Guo, Y.-L. Eur. J. Mass Spectrom. 2013, 19, 103.
[4] Gomez, M. J.; Gomez-Ramos, M. M.; Aguera, A.; Mezcua, M.; Herrera, S.; Fernandez-Alba, A. R. J. Chromatogr. A 2009, 1216, 4071.
[5] Li, Y.; Ruan, Q.; Li, Y.-L.; Ye, G.-Z.; Lu, X.; Lin, X.-H.; Xu, G.-W. J. Chromatogr. A 2012, 1255, 228.
[6] Wang, C.-Z.; Su, Y.; Guo, Y.-L. Chin. J. Org. Chem. 2009, 29, 948. (王呈仲, 苏越, 郭寅龙, 有机化学, 2009, 29, 948.)
[7] Su, Y.; Wang, C.-Z.; Guo, Y.-L. Acta Chim. Sinica 2009, 67, 546. (苏越, 王呈仲, 郭寅龙, 化学学报, 2009, 67, 546.)
[8] Liu, S.-H.; Wang, C.-Z.; Su, Y.; Guo, Y.-L. J. Instrum. Anal. 2010, 29(2), 126. (刘素红, 王呈仲, 苏越, 郭寅龙, 分析测试学报, 2010, 29(2), 126.)
[9] Wang, C.-Z.; Su, Y.; Li, D.; Cai, B.; Guo, Y.-L. Anal. Lett. 2010, 43, 2297.
[10] Zhu, F.-J.; Guo, Y.-L. Chin. J. Chem. 2010, 28, 1451.
[11] Su, Y.; Ji, H.-W.; Su, S.-B.; Guo, Y.-L. Anal. Test. Tech. Instrum. 2008, 14, 131. (苏越, 姬厚伟, 苏式兵, 郭寅龙, 分析测试技术与仪器, 2008, 14, 131.)
[12] Xian, F.; Hendrickson, C. L.; Marshall, A. G. Anal. Chem. 2012, 84, 708.
[13] Wang, H.; Wang, H.-Y.; Zhang, L.; Zhang, J.; Guo, Y.-L. Anal. Chim. Acta 2011, 690, 1.
[14] Johnson, J. V.; Yost, R. A. Anal. Chem. 1985, 57, 758.
[15] Wang, H.-Y.; Guo, Y.-L. J. Mass. Spectrom. 2011, 46, 856.
[16] Leng, J.-P.; Wang, H.-Y.; Zhang, L.; Zhang, J.; Guo, Y.-L. Chin. J. Chem. 2010, 28, 1751.
[17] Hong, S.-S.; Kang, W.-Y.; Su, Y.; Guo, Y.-L. Chin. J. Chem . 2013, 31, 1329.
[18] Lu, S.-S.; Su, Y.; Guo, Y.-L. Eur. J. Mass Spectrom. 2011, 17(4), 353.
[19] Liu, S.-H.; Lu, S.-S.; Su, Y.; Guo, Y.-L. Chromatographia 2011, 74, 497.
[20] Portoles, T.; Pitarch, E.; Lopez, F. J.; Sancho, J. V.; Hernandez, F. J. Mass Spectrom. 2007, 42, 1175.
[21] Cervera, M. I.; Portoles, T.; Pitarch, E.; Beltran, J.; Hernandez, F. J. Chromatogr. A 2012, 1244, 168.
[22] Serrano, R.; Nacher-Mestre, J.; Portoles, T.; Amat, F.; Hernandez, F. Talanta 2011, 85, 877.
[23] Redeuil, K.; Smarrito-Menozzi, C.; Guy, P.; Rezzi, S.; Dionisi, F.; Williamson, G.; Nagy, K.; Renouf, M. J. Chromatogr. A 2011, 1218, 4678.
[24] Portoles, T.; Pitarch, E.; Lopez, F. J.; Hernandez, F. J. Chromatogr. A 2011, 1218, 303.
[25] Fan, R.-J.; Zhang, F.; Wang, H.-Y.; Zhang, L.; Zhang, J.; Zhang, Y.; Yu, C.-T.; Guo, Y.-L. Sci. China, Ser. B: Chem. 2013, 56, 1.
[26] The Pharmacopoeia Committee of the People's Republic of China, The Pharmacopoeia of the People's Republic of China, 1st ed., Chemical Industry Press, Beijing, 2010. (中华人民共和国药典(一部), 化学工业出版社, 北京, 2010.)
[27] Yi, M.-H.; Xiao, H.; Liang, Z.-Y. China Trop. Med. 2004, 4(3), 339. (易美华, 肖红, 梁振益, 中国热带医学, 2004, 4(3), 339.)
[28] Lin, J.-M.; He, W.; Wu, M.-M.; Wu, Z.; Fu, T.-H.; Xia, P.-G.; Chen, F.-L. J. Chin. Med. Mater. 2000, 23(8), 448. (林敬明, 贺巍, 吴旻明, 吴忠, 付庭焕, 夏平光, 陈飞龙, 中药材, 2000, 23(8), 448.)
[29] Luo, X.-Z.; Yu, J.-G.; Xu, L.-Z.; Yang, S.-L.; Feng, J.-D.; Ou, S.-L. China J. Chin. Mater. Med. 2001, 26(4), 262. (罗秀珍, 余竞光, 徐丽珍, 杨世林, 冯锦东, 欧淑玲, 中国中药杂志, 2001, 26(4), 262.)
[30] Morikawa, T.; Matsuda, H.; Toguchida, I.; Ueda, K.; Yoshikawa, M. J. Nat. Prod. 2002, 65(10), 1468.
[31] Shoji, N.; Umeyama, A.; Asakawa, Y.; Takemoto, T.; Nomoto, K.; Ohizumi, Y. J. Pharm. Sci. 1984, 73, 843.
[32] Adams, R. P.; Habte, M.; Park, S.; Dafforn, M. R. Biochem. Syst. Ecol. 2004, 32, 1137.
[33] Huang, W.-Q.; Hu, C.-J.; Li, X.-Y.; Li, X.-H.; Yang, Z.-K.; Xie, X.-Q.; Peng, K.-C. China Pharm. 2008, 17(5), 3. (黄勤挽, 胡昌江, 李兴迎, 李兴华, 杨宗锟, 谢秀琼, 彭克成, 中国药业, 2008, 17(5), 3.)
[34] Morteza-Semnani, K.; Saeedi, M. Flavour Fragr. J. 2005, 20, 332.
[35] Yang, B.; Li, X.-B. Cent. South Pharm. 2010, 8(11), 817. (阳波, 李湘斌, 中南药学, 2010, 8(11), 817.)
[36] Javidnia, K.; Miri, R.; Kamalinejad, M.; Mehdipour, A. R. J. Essent. Oil Res. 2006, 18, 86.
[37] Maia, J. G. S.; Andrade, E. H. A.; da Silva, A. C. M.; Oliveira, J.; Carreira, L. M. M.; Araujo, J. S. Flavour Fragr. J. 2005, 20, 474.
/
| 〈 |
|
〉 |