

Self-assembly of Tetraaniline-PEG-Tetraaniline Block Copolymer Thin Films
Received date: 2013-10-12
Online published: 2013-12-17
Supported by
Project supported by National Natural Science Foundation of China (No. 21174009).
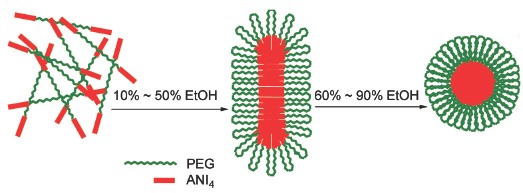
Amphiphilic rod-coil block copolymers containing polyethylene glycol and aniline oligomers can self-assemble into specific aggregation morphologies with different electrochemical performances, which have recently attracted more attention in many fields such as optoelectronic materials, electronics and so on. In this paper, the block copolymer of tetraaniline-polyethylene glycol-tetraaniline (ANI4-PEG-ANI4, PEG600, Mn=600) was prepared using tolylene 2,4-diisocyanate (TDI) as intermediate. At first, tetraaniline was synthesised by 4-aminodiphenyamine through oxidative coupling reaction in an acidic solution. Then, TDI was used to combine tetraaniline and PEG to prepare the triblock copolymer in the presence of N,N-dimethylformamide (DMF). 1H-NMR and FT-IR were combined to confirm the chemical structure of the resulting block copolymer. The self-assembly of the block copolymer thin films was studied in detail. ANI4-PEG-ANI4 block copolymer was dissolved in a mixing solvent with ethanol and DMF. Then the solution was coated on conductive glass ITO to prepare thin films with the same thickness. And the films were dried completely before use. Atomic force microscope (AFM), UV-Vis spectroscopy and cyclic voltammetry (CV) were used to study the self-assembly behaviors of ANI4-PEG-ANI4 block copolymer thin films. The influence of mixing ratio of ethanol/DMF and different annealing solvents on the self-assembly behaviors of ANI4-PEG-ANI4 block copolymer thin films were investigated. The annealing solvents included ethanol, methanol, chloroform and tetrahydrofuran. The results showed that by changing the mixing ratio of ethanol/DMF, the morphologies of the block copolymer thin films could be changed from spheric to rod-like morphologies. And by changing the annealing solvents, the copolymer thin films could self-assemble into such as spheric, rod-like and fibrillar morphologies. The different aggregation structures of the block copolymer thin films exhibited different electrochemical performances. So the aggregation structures of ANI4-PEG-ANI4 block copolymer thin films could be tuned nicely by changing the solvents and annealing solvents.
Key words: tetraaniline; polyethylene glycol; block copolymer; self-assembly; solvent annealing
Zhang Guangmeng , Yang Jiping , Chen Gong . Self-assembly of Tetraaniline-PEG-Tetraaniline Block Copolymer Thin Films[J]. Acta Chimica Sinica, 2014 , 72(1) : 83 -88 . DOI: 10.6023/A13101051
[1] Yang, H.; Xia, H.; Wang, G.-W.; Peng, J.; Qiu, F. J. Polym. Sci. Part A: Polym. Chem. 2012, 50, 5060.
[2] Tu, Y.-F.; Wan, X.-H.; Zhang, D.; Zhou, Q.-F.; Wu, C. J. Am. Chem. Soc. 2000, 122, 10201.
[3] Hu, F.-Z.; Chen, S.-D.; Li, H.; Sun, J.-J.; Sheng, R.-L.; Luo, T.; Cao, A.-M. Acta Chim. Sinica 2013, 71, 351. (胡方振, 陈圣典, 李慧, 孙景景, 盛瑞隆, 罗挺, 曹阿民, 化学学报, 2013, 71, 351.)
[4] Huang, L.-H.; Hu, J.; Lang, L.; Chen, X.-S.; Wei, Y.; Jing, X.-B. Macromol. Rapid Commun. 2007, 28, 1559.
[5] Zhu, Y.-M.; Zhang, Y.; Liu, Z.-L.; Lang, M.-D. Acta Chim. Sinica 2010, 68, 2449. (朱亚明, 张琰, 刘子路, 郎美东, 化学学报, 2010, 68, 2449.)
[6] Minich, E. A.; Nowak, A. P.; Deming, T. J.; Pochan, D. J. Polymer 2004, 45, 1951.
[7] Yin, B.-B.; Jiang, C.-Y.; Wang, Y.-G.; La, M.; Liu, P.; Deng, W.-J. Synth. Met. 2010, 160, 432.
[8] Chen, L.-X.; Weng, S.-H.; Zhou, J.-Z.; Lin, Z.-H. Chem. J. Chin. Univ. 2010, 31, 790. (陈丽娴, 翁少煌, 周剑章, 林仲华, 高等学校化学学报, 2010, 31, 790.)
[9] Xue, B.; Li, H.; Zhang, L.-L.; Peng, J. Thin Solid Films 2010, 518, 6107.
[10] Lu, F.-L.; Wudl, F.; Novak, M.; Heeger, A. J. J. Am. Chem. Soc. 1986, 108, 8311.
[11] Zhang, Z.-Y.; Yang, J.-P. Acta Chim. Sinica 2011, 69, 1247. (张增阳, 杨继萍, 化学学报, 2011, 69, 1247.)
[12] Deepam, M.; Ahmad, S.; Sood, K. N.; Alam, J.; Ahmad, S.; Srivastava, A. K. Electrochim. Acta 2007, 52, 7453.
[13] Zhang, Z.-Y.; Yang, J.-P. Rare Metals 2011, 30, 563.
[14] Chen, C.; Yu, C. H.; Cheng, Y. C.; Yu, P. H. F.; Cheung, M. K. Biomaterials 2006, 27, 4804.
[15] Yang, Z.-F.; Wang, X.-T.; Yang, Y.-K.; Liao, Y.-G.; Wei, Y.; Xie, X.-L. Langmuir 2010, 26, 9386.
[16] Hu, J.; Zhuang, X.-L.; Huang, L.-H.; Lang, L.; Chen, X.-S.; Wei, Y.; Jing, X.-B. Langmuir 2008, 24, 13376.
[17] Lu, W.-T.; Yang, J.-P. Acta Chim. Sinica 2012, 71, 121. (卢伟涛, 杨继萍, 化学学报, 2012, 71, 121.)
[18] Driscoll, S. O.; Demirel, G.; Farrell, R. A.; Fitzgerald, T. G.; Mahony, C. O.; Holmes, J. D.; Morris, M. A. Polym. Adv. Technol. 2011, 22, 915.
[19] Huang, W.-H.; Chen, P. Y.; Tung, S. H. Macromolecules 2012, 45, 1562.
[20] Albert, J. N. L.; Epps III, T. H. Mater. Today 2010, 13, 24.
[21] Park, S.; Wang, J.-Y.; Kim, B.; Chen, W.; Russell, T. P. Macromolecules 2007, 40, 9059.
/
| 〈 |
|
〉 |