

A Label-free Immunosensor based on Ionic Liquids Modified Mesoporous Silica for Simultaneous Determination of Two Tumor Markers
Received date: 2013-10-14
Online published: 2013-12-12
Supported by
Project supported by the Natural Science Foundation of China (Nos. 20905041 and 21075073) and the Doctoral Foundation of Shandong Province (No. BS2011SW008).
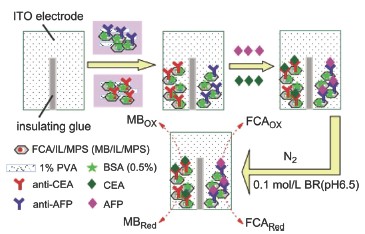
A label-free electrochemical immunoassay strategy was proposed for the simultaneous detection of two tumor markers, carcinoembryonic antigen (CEA) and α-fetoprotein (AFP). The functional mesoporous silica was synthesized for the construction of the label-free immunosensor. The Si-OH groups on the external surface of the mesoporous silica were terminated by trimethylchlorosilane (TMCS), while the Si-OH groups on the internal pore walls were modified with amino groups. The electrochemical substrates of ferrocenecarboxylic acid (FCA) and methylene blue (MB) were co-immobilized inside the channels of the ionic liquids (ILs) modified mesoporous silica (MPS), respectively. The monoclonal antibody of CEA (anti-CEA) and the monoclonal antibody of AFP (anti-AFP) were respectively co-immobilized inside the materials of FCA-IL-MPS and MB-IL-MPS. The ITO slide was separated lengthways into two uniform parts by insulation glue so as to avoid the cross-talk of the two portions. Finally, the suspension solutions were coated respectively onto the different areas of indium-tin oxide (ITO) electrode. The double-analyte immunosensor was constructed by the probes of CEA and AFP onto the different areas of ITO. When the double-analyte immunosensor was dipped into the sample solution, the antigens of CEA and AFP reacted with their corresponding monoclonal antibodies on the different area of the modified ITO electrode. After the immunological reaction, the nonconductive immunoconjugates formed inside the MPS channels. With the increasing concentrations of CEA and AFP antigens, the spatial blocking and impedance on the sensor surface increased, thus the electric response transfer from the solution to the electrode surface was blocked and the DPV currents decreased. The electrochemical signals for CEA were detected by using FCA as the electron mediator. The electrochemical signals for AFP were detected by using MB as the electrochemical substrates. Then, the simultaneous detection of CEA and AFP could be achieved. The electrode modification process was further characterized by cyclic voltammetric measurements and electrochemical impedance spectroscopy. IL units inside the mesopores could promote the electron transportation through the pore channel. To clarify the adsorption of FCA and MB into the mesopores of ILs-modified MPS, the IR spectra were recorded. The linear ranges of CEA and AFP were 0.5~80 ng·mL-1 and 0.5~100 ng·mL-1 with the detection limits of 0.1 ng·mL-1 and 0.1 ng·mL-1 (S/N=3), respectively. The fabricated immunosensor shows appropriate sensitivity because of the good electric conductivity of ILs. It is an alternative to the multianalyte detection of antigens or other bioactive molecules.
Lin Jiehua , Zhang Huihui , Shao Meijia . A Label-free Immunosensor based on Ionic Liquids Modified Mesoporous Silica for Simultaneous Determination of Two Tumor Markers[J]. Acta Chimica Sinica, 2014 , 72(2) : 241 -245 . DOI: 10.6023/A13101057
[1] Wu, J.; Fu, Z. F.; Yan, F.; Ju, H. X. Trends Anal. Chem. 2007, 26, 679.
[2] Wilson, M. S.; Nie, W. Y. Anal. Chem. 2006, 78, 6476.
[3] Wu, J.; Yan, Y. T.; Yan, F.; Ju, H. X. Anal. Chem. 2008, 80(15), 6072.
[4] Yang, Z. J.; Liu, H.; Zong, C.; Yan, F.; Ju, H. X. Anal. Chem. 2009, 81, 5484.
[5] Tlili, C.; Myunga, N. V.; Shettyb, V.; Mulchandani, A. Biosens. Bioelectron. 2011, 26, 4382.
[6] Luo, Y.; Chen, M.; Wen, Q. J.; Zhao, M.; Zhang, B.; Li, X. Y.; Wang, F.; Huang, Q.; Yao, C. Y.; Jiang, T. L.; Cai, J. R.; Fu, W. L. Clin. Chem. 2006, 52, 2273.
[7] Lee, H. J.; Namkoong, K.; Cho, E. C.; Ko, C.; Park, J. C.; Lee, S. S. Biosens. Bioelectron. 2009, 24, 3120.
[8] Zhang, J.; Lang, H. P.; Huber, F.; Bietsch, A.; Grange, W.; Certa, U.; Mckendry, R.; Güntherodt, H. J.; Hegner, M.; Gerber, C. Nat. Nanotechnol. 2006, 1, 214.
[9] Nasef, H.; Beni, V.; Ozalp, V. C.; O'Sullivan, C. K. Anal. Bioanal. Chem. 2010, 396, 2565.
[10] Loyprasert, S.; Thavarungkui, P.; Asawatreatanakul, P.; Wongkittisuksa, B.; Limsakul, C.; Kanatharana, P. Biosens. Bioelectron. 2008, 24, 78.
[11] Zhang, D. D.; Peng, Y. G.; Qi, H. G.; Gao, Q.; Zhang, C. X. Biosens. Bioelectron. 2010, 25, 1088.
[12] Escosura-Muñiz, A. D. L.; Merkoçi, A. Electrochem. Commun. 2010, 12, 859.
[13] Bayley, H.; Cremer, P. S. Nature 2001, 413, 226.
[14] Martin, C. R.; Siwy, Z. Nat. Mater. 2004, 3, 284.
[15] Lin, J. H.; Wei, Z. J.; Chu, P. F. Anal. Biochem. 2012, 421, 97.
[16] Lin, J. H.; Wei, Z. J.; Zhang, H. H.; Shao, M. J. Biosens. Bioelectron. 2013, 41, 342.
[17] Lin, J. H.; Wei, Z. J.; Mao, C. M. Biosens. Bioelectron. 2011, 29, 40.
[18] Zhao, D. Y.; Huo, Q. S.; Feng, J. L.; Chmelka, B. F.; Stucky, G. D. J. Am. Chem. Soc. 1998, 120, 6024.
[19] Zhao, D. Y.; Feng, J. L.; Huo, Q. S.; Melosh, N.; Fredrickson, G. H.; Chmelka, B. F.; Stucky, G. D. Science 1998, 279, 548.
[20] Lin, J. H.; Zhang, H. H.; Chu, P. F. Acta Chim. Sinica 2012, 70, 2372. (林洁华, 张慧慧, 楚鹏飞, 化学学报, 2012, 70, 2372.)
[21] Sun, J. M.; Ma, D.; Zhang, H.; Liu, X. M.; Han, X. W.; Bao, X. H.; Weinberg, G.; Pfander, N.; Su, D. S. J. Am. Chem. Soc. 2006, 128, 15756.
[22] Buzzeo, M. C.; Evans, R. G.; Compton, R. G. ChemPhysChem 2004, 5, 1106.
[23] Endres, F. Zeitschrift Fur Physikalische Chemie. 2004, 218, 255.
[24] Lin, J. H.; Qu, W.; Zhang, S. S. Anal. Biochem. 2007, 360, 288.
/
| 〈 |
|
〉 |