

Spectral Study on the Photoreduction of Cytochrome c under Macromolecular Crowding
Received date: 2013-10-04
Online published: 2013-12-27
Supported by
Project supported by the National Natural Science Foundation of China (No. 21271036, 20871024) and Science & Technology Project of Liaoning Province Department of Education (No. L2013470, 2013471).
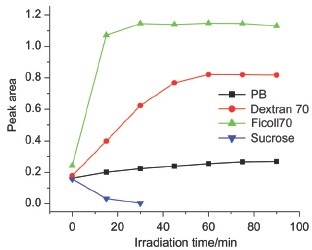
The ferric Cyt c can be photoreduced to the ferrous state by photoexcitation in dilute solution. However, these researches neglected the high crowded conditions in cell environment. In this work, we applied UV-Vis absorption, synchronous fluorescence and CD spectra to study the photoreduction process of Cyt c and its dependence on the external environment under macromolecular crowded conditions. The UV-Vis absorption spectrum data showed that with addition of Dextran70 or Ficoll70 the hydrophobic microenvironment of heme in Cyt c was changed and the photoreduction process of Cyt c was facilitated. In addition, the photoreduction extent was much larger in Ficoll70 solution than that in Dextran70. Under macromolecular crowded conditions, we used four wavelengths of light to irradiate Cyt c samples and found that the 280 nm light was favored for the reduction. The optimum temperatures of photoreduction process were 37 ℃ and 25 ℃ in the presence of Dextran70 and Ficoll70, respectively. The concentration of macromolecular crowding reagents had massive influence on the photoreduction process. Photoreduction extent showed an increase with rising the concentration of Dextran70 and the optimum concentration of Ficoll70 for the photoreduction was 100 g·L-1. The photoreduction process was promoted by adding external amino acids and the relative rates of photoreduction with amino acids followed the order Phe> Tyr> Trp> Met. Synchronous fluorescence spectra data suggested that Dextran70 and Ficoll70 were able to emit fluorescence which was absorbed by porphyrin. The CD spectra results of Cyt c after irradiation illustrated that the secondary structure of protein varied with α-helix decreased, and β-sheet increased in dilute solution, but remained unchanged after irradiation under macromolecular crowding solutions. Based on the data, macromolecular crowded conditions could maintain a stable structure of Cyt c and promote its photoinduced electronic transfer and reduction process.
Liu Yanwei , Cao Hongyu , Tang Qian , Zheng Xuefang . Spectral Study on the Photoreduction of Cytochrome c under Macromolecular Crowding[J]. Acta Chimica Sinica, 2014 , 72(2) : 246 -252 . DOI: 10.6023/A13101029
[1] Ellis, R. J. Trends Biochem. Sci. 2001, 26, 597.
[2] Perham, M.; Stagg, L.; Wittung-Stafshede, P. FEBS Lett. 2007, 581, 5065.
[3] Zimmerman, S. B.; Minton, A. P. Ann. Rev. Biophys. Biomol. Struct. 1993, 22, 27.
[4] Minton, A. P. Biophys. J. 2005, 88, 971.
[5] Minton, A. P. J. Pharm. Sci. 2005, 94, 1668.
[6] Zhou, H. X. J. Mol. Recognit. 2004, 17, 368.
[7] Zhu, J.; He, H. W.; Li, S. Tsinghua Sci. Tech. 2008, 13, 454.
[8] Lin, Y. W.; Huang, Z. X. World Sci-tech. R & D 2006, 28, 8. (林英武, 黄仲贤, 世界科技研究与发展, 2006, 28, 8.)
[9] Bazin, M.; Pierre, J.; Debey, P.; Santus, R. Eur. J. Biochem. 1982, 124, 539.
[10] Sakai, H.; Onuma, H.; Umeyama, M.; Takeoka, S.; Tsuchida, E. Biochemistry 2000, 39, 14595.
[11] Liang, X. Q.; Chen, G. F.; Zhang, X.; Liu, S. L.; Li, G. X. Photochem. Photobiol. B 2008, 90, 53.
[12] Vorkink, W. P.; Cusanovich, M. A. Photochem. Photobiol. 1974, 19, 205.
[13] Masuda, T.; Minemura, A.; Yamauchi, K.; Kondo, M. J. Radiat Res. 1980, 21, 149.
[14] Winterle, J. S.; Einarsdóttir, Ó. Photochem. Photobiol. 2006, 82, 711.
[15] Prusakov, V. E.; Steyer, J.; Parak, F. G. Biophys. J. 1995, 68, 2524.
[16] Zhou, H. W.; Cao, H. Y.; Tang, Q.; Zheng, X. F. Acta Chim. Sinica 2011, 69, 1559. (周华伟, 曹洪玉, 唐乾, 郑学仿, 化学学报, 2011, 69, 1559.)
[17] An, L. M.; Cao, H. Y.; Tang, Q.; Zheng, X. F. Chin. J. Inorg. Chem. 2012, 28, 1461. (安良梅, 曹洪玉, 唐乾, 郑学仿, 无机化学学报, 2012, 28, 1461.)
[18] Wallace, C. J.; Clark-Lewis, I. J. Biol. Chem. 1992, 267, 3852.
[19] Gu, X. T.; Wu, X. H.; Zhou, J. H.; Wei, S. H.; Liu, Y.; Feng, Y. Y. J. Nanjing Normal Univ. 2005, 28, 70. (顾晓天, 吴晓红, 周家宏, 魏少华, 刘颖, 冯玉英, 南京师范大学学报, 2005, 28, 70.)
[20] Yang, C. Y.; Li, M.; Fu, J.; Li, X. J. Anal. Sci. 2010, 26, 153. (杨昌英, 李敏, 付静, 李昕, 分析科学学报, 2010, 26, 153.)
[21] Mukherjee, S.; Waegele, M. M.; Chowdhury, P.; Lin, G.; Feng, G. J. Mol. Biol. 2009, 393, 227.
[22] Venturoli, D.; Rippe, B. Am. J. Physiol. 2005, 288, 605.
[23] Huang, H. C.; Jiang, Z. F.; Zhu, H. J. Chemistry 2007, 70, 501. (黄汉昌, 姜招峰, 朱宏吉, 化学通报, 2007, 70, 501.)
[24] Shen, X. C.; Liang, H.; He, X. W.; Wang, X. S. Chin. J. Anal. Chem. 2004, 32, 388. (沈星灿, 梁宏, 何锡文, 王新省, 分析化学, 2004, 32, 388.)
[25] Zhang, Y. J.; Tang, Q.; Cao, H. Y.; Zheng, X. F. Acta Phys. Chim. Sin. 2013, 29, 1785. (张玉姣, 唐乾, 曹洪玉, 郑学仿, 物理化学学报, 2013, 29, 1785.)
/
| 〈 |
|
〉 |