

Determination of pH Value and Acid-base Property of Ionic Liquid Aqueous Solutions
Received date: 2013-09-29
Online published: 2014-01-16
Supported by
Project supported by the National Natural Science Foundation of China (No. 51076169) and the Natural Science Foundation of Guangdong Province (No. 9251027501000001).
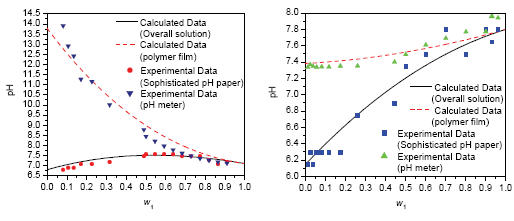
The ionic liquids (ILs) and their aqueous solutions have been considered as a new generation of absorption working substances for applications in gas separation and absorption refrigeration processes. It is important to understand the physicochemical properties of ionic liquids in aqueous solutions, such as chemical potential and acid-base properties before any industry application. In this study, we focused on the acid-base properties of the IL aqueous solutions by testing the pH values of the aqueous solutions of two ionic liquids, 1-ethyl-3-methylimidazolium acetate ([EMIM]Ac) and 1-hexyl-3-methylimidazolium chloride ([HMIM]Cl). All the tests were carried out at 25 ℃ and in a well controlled dry air surrounding. There were 16 water mass fractions for each of [EMIM]Ac and [HMIM]Cl aqueous solutions tested in range of 0.04 to 0.95 and 0.01 to 0.96, respectively. There, two methods, the sophisticated pH paper and the pH meter, were applied for the tests and the differences between the two test methods were detected. The test data from the two test methods and their differences were then evaluated with an aggregation film model and the activity theory for the film and aqueous solution phases. The results show that the difference of the pH values tested by the two methods is significant. The pH data from the sophisticated pH paper test present the bulk acid-base property of the IL aqueous solutions, while the pH meter can only reflect the hydrogen ion concentration in the IL aggregation film which was formed on the surface of the electrode of the pH meter. With analysis of acid-base reaction in the IL aqueous solution, a calculation model for the pH value of the bulk solution region was developed, and the data of the sophisticated pH paper test were correlated and the solution acid-base values were obtained. Based on analysis of the water species equilibrium in the IL aggregation film and the bulk aqueous solution phases and its effect on the concentration of hydrogen ions in the aggregation film, a method for calculation of the difference of pH values between the aggregation film and the bulk solution was proposed and the data from the pH meter were then fitted with the proposed calculation method. The results provide experimental data and a quantitative method for analysis of the acid-base property of IL aqueous solution systems.
Key words: ionic liquid; aqueous solution; pH value; acid-base property
Yang Cuilian , Huangfu Lixia , Su Hang , Guo Kaihua . Determination of pH Value and Acid-base Property of Ionic Liquid Aqueous Solutions[J]. Acta Chimica Sinica, 2014 , 72(4) : 495 -501 . DOI: 10.6023/A13091014
[1] Gao, X.; Fan, J.; Wang, X. L.; Zhang, Y. S. Acta Chim. Sinica 2013, 71(10), 1411. (高霞, 樊静, 王小龙, 张艳树, 化学学报, 2013, 71(10), 1411.)
[2] Huang, B.; Li, Z.; Wang, Y.; Zhang, Y.; Fang, Y. Acta Chim. Sinica 2008, 66(15), 1837. (黄宝华, 黎子进, 汪艳飞, 张煜, 方岩雄, 化学学报, 2008, 66(15), 1837.)
[3] Huo, C. D.; Wang, C. Chin. J. Org. Chem. 2013, 33, 2108. (霍聪德, 王程, 有机化学, 2013, 33, 2108.)
[4] Zhang, P.; Du, J.; Yang, F.; Zou, G.; Tang, J. Chin. J. Chem. 2005, 23(5), 581.
[5] Yokozeki, A.; Shiflett, M. B. Appl. Energy 2007, 84(12), 1258.
[6] Shiflett, M. B.; Yokozeki, A. US 20060197053, A1 2006 [Chem. Abstr. 2006, 2597199 A1].
[7] Wei, Z.; Wu, X. H.; Zheng, D. X.; Wang, J. Z.; Dong, L. J. Beijing Univ. Chem. Technol. (Nat. Sci. Ed.) 2010, 37(01), 9. (魏治, 武向红, 郑丹星, 王建召, 董丽, 北京化工大学学报(自然科学版), 2010, 37(01), 9.)
[8] Tian, T.; Zheng, D. X.; Wu, X. H.; Jiang, Y. R. J. Beijing Univ. Chem. Technol. (Nat. Sci. Ed.) 2008, 35(03), 27. (田涛, 郑丹星, 武向红, 蒋翼然, 北京化工大学学报(自然科学版), 2008, 35(03), 27.)
[9] Dong, L.; Zheng, D. X.; Wu, X. H.; Li, F.; Wei, Z. J. Eng. Thermophys. 2009, 30(08), 1271. (董丽, 郑丹星, 武向红, 李枫, 魏治, 工程热物理学报, 2009, 30(08), 1271.)
[10] Guan, T. T.; Sun, L.; Huangfu, L. X.; Guo, K. H. Chinese J. Low Temp. Phys. 2011, 33(03), 194. (关婷婷, 孙立, 皇甫立霞, 郭开华, 低温物理学报, 2011, 33(03), 194.)
[11] Sun, L.; Guo, K. H.; Huangfu, L. X. Chinese J. Low Temp. Phys. 2011, 33(05), 381. (孙立, 郭开华, 皇甫立霞, 低温物理学报, 2011, 33(05), 381.)
[12] Yang, T.; Bi, Y.; Guo, K. H. CIESC J. 2012, 63(10), 3152. (阳涛, 毕崟, 郭开华, 化工学报, 2012, 63(10), 3152.)
[13] Yuan, X.; Zhang, S.; Liu, J.; Lu, X. Fluid Phase Equilib. 2007, 257(2), 195.
[14] Pan, S. F.; Hu, G. X.; Lv, Y.; Zou, J. W.; Yu, Q. S. Acta Phys.-Chim. Sin. 2010, 26(9), 2494. (潘善飞, 胡桂香, 吕杨, 邹建卫, 俞庆森, 物理化学学报, 2010, 26(9), 2494.)
[15] Liang, R.; Yang, M. R.; Zhou, Q. X. Acta Phys.-Chim. Sin. 2010, 26(6), 1468. (梁蕊, 杨美荣, 周庆祥, 物理化学学报, 2010, 26(6), 1468.)
[16] Aggarwal, V. K.; Emme, I.; Mereu, A. Chem. Commun. 2002, 1612.
[17] Seddon, K. R. J. Chem. Technol. Biot. 1997, 68(4), 351.
[18] Fei, Z.; Zhao, D.; Geldbach, T. J.; Scopelliti, R.; Dyson, P. J. Chem. -Eur. J. 2004, 10(19), 4886.
[19] Thomazeau, C.; Olivier-Bourbigou, H.; Magna, L.; Luts, S.; Gilbert, B. J. Am. Chem. Soc. 2003, 125(18), 5264.
[20] Yang, Y.; Kou, Y. Chem. Commun. 2004, 226.
[21] Chen, X. W.; Song, H. B.; Chen, P.; Wang, F. R.; Qian, Y.; Li, X. H. Acta Chim. Sinica. 2012, 70, 770. (陈学伟, 宋红兵, 陈鹏, 王芙蓉, 钱宇, 李雪辉, 化学学报, 2012, 70, 770.)
[22] Ober, C. A.; Gupta, R. B. Ind. Eng. Chem. Res. 2012, 51(6), 2524.
[23] Guo, K.; Bi, Y.; Sun, L.; Su, H.; Hungpu, L. J. Chem. Eng. Data 2012, 57(8), 2243.
[24] Su, H.; Guo, K. H.; Huangfu, L. X.; Sun, L. J. Refrig. 2013, 34(3), 24. (粟航, 郭开华, 皇甫立霞, 孙立, 制冷学报, 2013, 34(3), 24.)
[25] Guo, K.; Bi, Y.; Sun, L.; Su, H.; Hungpu, L. J. Chem. Eng. Data 2012, 57(8), 2243.
[26] Singh, T.; Kumar, A. Colloids Surf. A 2008, 318(1), 263
[27] Fu, S. Z.; Chen, Q. D.; Shen, X. H. Acta Phys.-Chim. Sin. 2011, 27(8), 1913. (付素珍, 陈庆德, 沈兴海, 物理化学学报, 2011, 27(8), 1913.)
[28] Sun, L.; Guo, K. H.; Huangfu, L. X. Chin. J. Low Temp. Phys. 2011, 33(6), 467. (孙立, 郭开华, 皇甫立霞, 低温物理学报, 2011, 33(6), 467.)
[29] Long, Y. H.; Xiang, M. L.; Gao, Y. H. J. Chongqing Institute Technol. 2001, 15(05), 98. (龙彦辉, 向明礼, 高彦荷, 重庆工学院学报, 2001, 15(05), 98.)
[30] http://www.chem.wisc.edu/areas/reich/pkatable/index.htm, 2013.7.22[Z].
[31] Hou, H.; Huang, Y. R.; Wang, S. Z.; Bai, B. F. Acta Phys.-Chim. Sin. 2011, 27(11), 2512. (侯海云, 黄银蓉, 王升泽, 白博峰, 物理化学学报, 2011, 27(11), 2512.)
[32] Ning, H.; Hou, M.; Mei, Q.; Liu, Y.; Yang, D.; Han, B. Sci. China Chem. 2012, 55(08), 1509.
/
| 〈 |
|
〉 |