

Single Molecule Force Spectroscopy Investigation on Na2SO4-inducedConformational Transition of Single PNIPAM Chains
Received date: 2013-12-28
Online published: 2014-01-22
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20640420622, 91127031, 21221063).
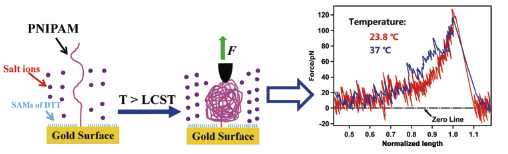
In this study, the sodium sulfate (Na2SO4)-induced conformational transition of a single PNIPAM chain, a model system for the investigation of protein folding, was studied by using atomic force microscopy (AFM) based single-molecule force spectroscopy (SMFS). Before the SMFS experiments, thermal-responsive poly(N-isopropylacrylamide) (PNIPAM) molecules were attached individually onto gold surfaces for SMFS experiments. By covalently grafting sulfydryl- functionalized PNIPAM into the isolated defects of freshly prepared self-assembled monolayers (SAMs) of dithiothreitol (DTT) on gold substrates, the grafting density of PNIPAM molecules could be conveniently controlled and the substrate-induced conformational changes have also been prevented effectively. During the SMFS experiments, the AFM tip was gently brought in contact with the sample surfaces during which part of the polymer chain was adsorbed onto the tip surface, forming a connective bridge in-between. And then, the grabbed polymer chain has been manipulated repeatedly under different extension length until the molecule detached from the AFM tip. Characteristic smooth-and sawtooth-pattern force curves were obtained before and after the conformational transition of a single PNIPAM chain, respectively. It was demonstrated that the smooth-pattern force curves corresponded to the pulling of the random coiled structure of single PNIPAM chains, while the sawtooth-pattern force curves corresponded to the unraveling of the collapsed globule structure. Besides, a consecutive two-step collapsing process (low-and high-temperature collapsing step) of the conformational transition of a single PNIPAM chain was detected, for the first time, in our SMFS experiments. It was found that more compact collapsed structures formed in the high-temperature collapsing step, which may due to the cooperative effect of the weakening of hydrogen bonding (between PNIPAM chains and water molecules) and increasing of hydrophobic interaction of the polymer chains. Our results show that AFM-based SMFS is a powerful method that complementary to other ensemble measurements in the determination of conformation transition of thermal-responsive polymers.
Xue Yurui , Zhang Wenke . Single Molecule Force Spectroscopy Investigation on Na2SO4-inducedConformational Transition of Single PNIPAM Chains[J]. Acta Chimica Sinica, 2014 , 72(4) : 481 -486 . DOI: 10.6023/A13121281
[1] Galaev, I. Y.; Mattiasson, B. Trends Biotechnol. 2000, 17, 335.
[2] Gil, E. S.; Hudson, S. M. Prog. Polym. Sci. 2004, 29, 1173.
[3] Kumar, A.; Srivastava, A.; Galaev, I. Y.; Mattiasson, B. Prog. Polym. Sci. 2007, 32, 1205.
[4] Stuart, M. A. C.; Huck, W. T. S.; Genzer, J.; MÜller, M.; Ober, C.; Stamm, M.; Sukhorukov, G. B.; Szleifer, I.; Tsukruk, V. V.; Urban, M.; Winnik, F.; Zauscher, S.; Luzinov, I.; Minko, S. Nat. Mater. 2010, 9, 101.
[5] Schild, H. G. Prog. Polym. Sci. 1992, 17, 163.
[6] Wu, C.; Zhou, S. Phys. Rev. Lett. 1996, 77, 3053.
[7] Wu, C.; Wang, X. Phys. Rev. Lett. 1998, 80, 4092.
[8] Zhang, W.; Zou, S.; Wang, C.; Zhang, X. J. Phys. Chem. B 2000, 104, 10258.
[9] Zhang, G. Macromolecules 2004, 37, 6553.
[10] Schmaljohann, D. Adv. Drug Delivery Rev. 2006, 58, 1655.
[11] Cui, S.; Pang, X.; Zhang, S.; Yu, Y.; Ma, H.; Zhang, X. Langmuir 2012, 28, 5151.
[12] Kutnyanszky, E.; Embrechts, A.; Hempenius, M. A.; Vancso, G. J. Chem. Phys. Lett. 2012, 535, 126.
[13] Zhang, Y.; Furyk, S.; Bergbreiter, D. E.; Cremer, P. S. J. Am. Chem. Soc. 2005, 127, 14505.
[14] Ishida, N.; Biggs, S. Macromolecules 2007, 40, 9045.
[15] Du, H.; Wickramasinghe, R.; Qian, X. J. Phys. Chem. B 2010, 114, 16594.
[16] Amiya, T.; Hirokawa, Y.; Hirose, Y.; Li, Y.; Tanaka, T. J. Chem. Phys. 1987, 86, 2375.
[17] Zhang, G.; Wu, C. Phys. Rev. Lett. 2001, 86, 822.
[18] Zhang, G.; Wu, C. J. Am. Chem. Soc. 2001, 123, 1376.
[19] Wang, T.; Liu, G.; Zhang, G.; Craig, V. S. J. Langmuir 2012, 28, 1893.
[20] Pang, X.; Wang, K.; Cui, S. Polymer 2013, 54, 3737.
[21] Rief, M.; Gautel, M.; Oesterhelt, F.; Fernandez, J. M.; Gaub, H. E. Science 1997, 276, 1109.
[22] Ye, X.; Lu, Y.; Shen, L.; Ding, Y.; Liu, S.; Zhang, G.; Wu, C. Macromolecules 2007, 40, 4750.
[23] Halperin, A.; Zhulina, E. B. Europhys. Lett. 1991, 15, 417.
[24] Halperin, A.; Goldbart, P. M. Phys. Rev. Lett. 2000, 61, 565.
[25] Merkel, R. Phys. Rep. 2001, 346, 343.
[26] Zhang, W.; Zhang, X. Prog. Polym. Sci. 2003, 28, 1271.
[27] Neuman, K. C.; Nagy, A. Nat. Methods 2008, 5, 491.
[28] Tan, X.; Yu, Y.; Liu, K.; Xu, H.; Liu, D.; Wang, Z.; Zhang, X. Langmuir 2012, 28, 9601.
[29] Zhang, W.; Kou, X.-L.; Zhang, W.-K. Chem. J. Chin. Univ. 2012, 33, 861. (张薇, 寇晓龙, 张文科, 高等学校化学学报, 2012, 33, 861.)
[30] Wang, Q.; Sun, X.-L.; Yang, X.-H.; Wang, K.-M.; Wu, C.-L.; Chen, T. Chem. J. Chin. Univ. 2012, 33, 1401. (王青, 孙小兰, 羊小海, 王柯敏, 吴春玲, 陈桐, 高等学校化学学报, 2012, 33, 1401.)
[31] Liu, K.; Zheng, X.; Samuel, A. Z.; Ramkumar, S. G.; Ghosh, S.; Tan, X.; Wang, D.; Shuai, Z.; Ramakrishnan, S.; Liu, D.; Zhang, X. Langmuir 2013, 29, 14438.
[32] Zhang, W.; Barbagallo, R.; Madden, C.; Roberts, C. J.; Woolford, A.; Allen, S. Nanotechnology 2005, 16, 2325.
[33] MÜller, D. J.; Dufrêne, Y. F. Nat. Nanotechnol. 2008, 3, 261.
[34] Liu, N.; Peng, B.; Lin, Y.; Su, Z.; Niu, Z.; Wang, Q.; Zhang, W.; Li, H.; Shen, J. J. Am. Chem. Soc. 2010, 132, 11036.
[35] Liu, K.; Song, Y.; Feng, W.; Liu, N.; Zhang, W.; Zhang, X. J. Am. Chem. Soc. 2011, 133, 3226.
[36] Zhang, W.; LÜ, X.; Zhang, W.; Shen, J. Langmuir 2011, 27, 15008.
[37] Liu, N.; Zhang, W. ChemPhysChem 2012, 13, 2238.
[38] Shi, Z.-M.; Song, Y.; Lu, F.; Zhou, T.-Y.; Zhao, X.; Zhang, W.-K.; Li, Z.-T. Acta Chim. Sinica 2013, 71, 51. (施朱明, 宋宇, 陆方, 周天佑, 赵新, 张文科, 黎占亭, 化学学报, 2013, 71, 51.)
[39] Friggeri, A.; Schönherr, H.; van Manen, H.-J.; Huisman, B.-H.; Vancso, G. J.; Huskens, J.; van Veggel, F. C. J. M.; Reinhoudt, D. N. Langmuir 2000, 16, 7757.
[40] Zou, S.; Ma, Y.; Hempenius, M. A.; Schönherr, H.; Vancso, G. J. Langmuir 2004, 20, 6278.
[41] Zhao, Y.; Guo, K.; Wang, C.; Wang, L. Langmuir 2010, 26, 8966.
[42] Zhang, Y.; Furyk, S.; Sagle, L. B.; Cho, Y.; Bergbreiter, D. E.; Cremer, P. S. J. Phys. Chem. C 2007, 111, 8916.
[43] Halperin, A.; Zhulina, E. B. Macromolecules 1991, 24, 5393.
[44] Gunari, N.; Balazs, A. C.; Walker, G. C. J. Am. Chem. Soc. 2007, 129, 10046.
[45] Wang, X.; Qiu, X.; Wu, C. Macromolecules 1998, 31, 2972.
[46] Katsumoto, Y.; Tanaka, T.; Sato, H.; Ozaki, Y. J. Phys. Chem. A 2001, 106, 3429.
[47] Cho, E. C.; Lee, J.; Cho, K. Macromolecules 2003, 36, 9929.
[48] Okada, Y.; Tanaka, F. Macromolecules 2005, 38, 4465.
[49] Geukens, B.; Meersman, F.; Nies, E. J. Phys. Chem. B 2008, 112, 4474.
[50] Zhou, K.; Lu, Y.; Li, J.; Shen, L.; Zhang, G.; Xie, Z.; Wu, C. Macromolecules 2008, 41, 8927.
[51] Creczynski-Pasa, T. B.; Millone, M. A. D.; Munford, M. L.; de Lima, V. R.; Vieira, T. O.; Benitez, G. A.; Pasa, A. A.; Salvarezza, R. C.; Vela, M. E. Phys. Chem. Chem. Phys. 2009, 11, 1077.
[52] Butt, H.-J.; Jaschke, M. Nanotechnology 1995, 6, 1.
[53] Zhang, W.; Wang, C.; Zhang, X. Chin. Sci. Bull. 2003, 48, 1113. (张文科, 王驰, 张希, 科学通报, 2003, 48, 1113.)
[54] Zhang, W. K. Ph.D. Dissertation, Jilin University, Changchun, 2002. (张文科, 博士论文, 吉林大学, 长春, 2002.)
[55] Jiang, B.; Tang, X. Y.; Song, Y.; Zhang, W. K. Scientia Sinica Chimica 2013, 43, 1780. (姜博, 汤孝妍, 宋宇, 张文科, 中国科学: 化学, 2013, 43, 1780.)
/
| 〈 |
|
〉 |