

Synthesis and Gas-Sensing Properties of ZnO Porous Microflowers
Received date: 2013-12-04
Online published: 2014-02-17
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21173115, 20873057).
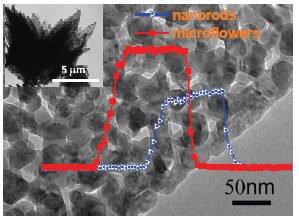
Three-dimensional (3D) porous nanomaterials with large surface area and abundant surface defects have promising potential in gas sensing due to the following merits: (i) large contact area of gaseous species with the materials, and more adsorption sites on the highly defective surface, (ii) good accessibility to gaseous species of the 3D open structure, (iii) fast electron transport among the 3D network. Rational design and controlled synthesis of the 3D porous nanomaterials with specific morphology and microstructure are essential for improving the performance of gas sensing. In this work, 3D ZnO porous microflowers were prepared by directly calcining the zinc-based flowerlike precursor at 400 ℃ in air. The precursor was preformed through a simple coprecipitation method, i.e., by refluxing the aqueous solution of zinc nitrate and co-precipitators of hexamethylenetetramine and oxalic acid at 90 ℃ for 4 h. The unique ZnO porous microflowers were composed of porous nanosheets of 10~50 nanometers in thickness and 1~2 micrometers in width, which inherited from the zinc-based precursor except for the randomly distributed pores on the nanosheet "petals". The 3D porous microstructures endowed ZnO with large specific surface area of 31.3 m2·g-1 and abundant surface defects. ZnO porous microflowers were used as active materials to fabricate gas sensors, which exhibited low working temperature, high sensitivity and fast response (recovery) characteristic against ethanol vapor, at the advanced level in comparison with the reported ZnO-based gas sensors for ethanol. The superior performance of the gas sensors could be attributed to the unique microstructures of the ZnO porous nanomaterials. In addition, the sensitivity of the gas sensors showed an exponential relationship with the concentration of ethanol vapor, indicating their capability of quantitative detection within the ethanol volume ratio of 1×10-6~500×10-6. Together with the superb sensing performance, simple preparation processing and low cost, ZnO porous microflowers have promising application prospect in gas sensing area.
Xiong Jingfang , Xiao Pei , Wu Qiang , Wang Xizhang , Hu Zheng . Synthesis and Gas-Sensing Properties of ZnO Porous Microflowers[J]. Acta Chimica Sinica, 2014 , 72(4) : 433 -439 . DOI: 10.6023/A13121212
[1] Huang, M. H.; Mao, S.; Feick, H.; Yan, H. Q.; Wu, Y. Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. D. Science 2001, 292, 1897.
[2] Wang, Z. L.; Song, J. H. Science 2006, 312, 242.
[3] Wang, Z. L. Adv. Mater. 2012, 24, 4632.
[4] Law, M.; Greene, L. E.; Johnson, J. C.; Saykally, R. J.; Yang, P. D. Nat. Mater. 2005, 4, 455.
[5] Gao, T.; Wang, T. H. Appl. Phys. A 2005, 80, 1451.
[6] Feng, X. J.; Feng, L.; Jin, M. H.; Zhai, J.; Jiang, L.; Zhu, D. B. J. Am. Chem. Soc. 2004, 126, 62.
[7] Vayssieres, L. Adv. Mater. 2003, 15, 464.
[8] Arnold, M. S.; Avouris, P.; Pan, Z. W.; Wang, Z. L. J. Phys. Chem. B 2003, 107, 659.
[9] Kuo, C. L.; Kuo, T. J.; Huang, M. H. J. Phys. Chem. B 2005, 109, 20115.
[10] Li, J.; Fan, H.; Jia, X. J. Phys. Chem. C 2010, 114, 14684.
[11] Greene, L. E.; Law, M.; Goldberger, J.; Kim, F.; Johnson, J. C.; Zhang, Y. F.; Saykally, R. J.; Yang, P. D. Angew. Chem., Int. Ed. 2003, 42, 3031.
[12] Pan, Z. W.; Dai, Z. R.; Wang, Z. L. Science 2001, 291, 1947.
[13] Zheng, J. H.; Zhang, X. K.; Lu, H. F. Acta Chim. Sinica 2011, 69, 2434. (郑建华, 张晓凯, 卢慧粉, 化学学报, 2011, 69, 2434.)
[14] Bai, H. Y.; Bao, J. C.; Dai, Z. H.; Liu, K. Acta Chim. Sinica 2008, 66, 1786. (白红艳, 包建春, 戴志晖, 刘可, 化学学报, 2008, 66, 1786.)
[15] Zhang, N.; Yu, K.; Li, Q.; Zhu, Z. Q.; Wan, Q. J. Appl. Phys. 2008, 103, 104305.
[16] Son, J. Y.; Lim, S. J.; Cho, J. H.; Seong, W. K.; Kim, H. Appl. Phys. Lett. 2008, 93, 053109.
[17] Law J. B. K.; Thong, J. T. L. Nanotechnology 2008, 19, 205502.
[18] Jing, Z. H.; Zhan, J. H. Adv. Mater. 2008, 20, 4547.
[19] Zuruzi, A. S.; MacDonald, N. C.; Moskovits, M.; Kolmakov, A. Angew. Chem., Int. Ed. 2007, 46, 4298.
[20] Chen, M.; Wang, Z.; Han, D.; Gu, F.; Guo, G. J. Phys. Chem. C 2011, 115, 12763.
[21] Zhang, H.; Wu, J. B.; Zhai, C. X.; Du, N.; Ma, X. Y.; Yang, D. R. Nanotechnology 2007, 18, 455604.
[22] Lee, J. H. Sens. Actuators, B 2009, 140, 319.
[23] Fu, M.; Zhou, J.; Xiao, Q. F.; Li, B.; Zong, R. L.; Chen, W.; Zhang, J. Adv. Mater. 2006, 18, 1001.
[24] Ding, G. Q.; Shen, W. Z.; Zheng, M. J.; Fan, D. H. Appl. Phys. Lett. 2006, 88, 103106.
[25] Zhang, W. X.; Yanagisawa, K. Chem. Mater. 2007, 19, 2329.
[26] Song, R. Q.; Xu, A. W.; Deng, B.; Chen, G. Y. Adv. Funct. Mater. 2007, 17, 296.
[27] Gui, Z.; Liu, J.; Wang, Z. Z.; Song, L.; Hu, Y.; Fan, W. C.; Chen, D. Y. J. Phys. Chem. B 2005, 109, 1113.
[28] Xiong, J. F.; Shen, H.; Mao, J. X.; Qin, X. T.; Xiao, P.; Wang, X. Z.; Wu, Q.; Hu, Z. J. Mater. Chem. 2012, 22, 11927.
[29] Korotcenkov, G.; Han, S. D.; Cho, B. K.; Brinzari, V. Critical Rev. Solid State Mater. Sci. 2009, 34, 1.
[30] Waitz, T.; Becker, B.; Wagner, T.; Sauerwald, T.; Kohl, C. D.; Tiemann, M. Sens. Actuators, B 2010, 150, 788.
[31] Wu, Q.; Chen, J. X.; Zhang, F.; Xiao, P.; Lu, Y. N.; Wang, X. Z.; Hu, Z. CrystEngComm 2012, 14, 3397.
[32] Liu, J. F.; Wang, X.; Peng, Q.; Li, Y. D. Adv. Mater. 2005, 17, 764.
[33] Wang, J. X.; Sun, X. W.; Yang, Y.; Huang, H.; Lee, Y. C.; Tan, O. K.; Vayssieres, L. Nanotechnology 2006, 17, 4995.
[34] Wang, X. Z.; Liu, W.; Liu, J. R.; Wang, F. L.; Kong, J.; Qiu, S.; He, C. Z.; Luan, L. Q. ACS Appl. Mater. Interfaces 2012, 4, 817.
[35] Wang, L. W.; Kang, Y. F.; Liu, X. H.; Zhang, S. M.; Huang, W. P.; Wang, S. R. Sens. Actuators, B 2012, 20, 237.
[36] Chen, J.; Li, J.; Li, J. H.; Xiao, G. Q.; Yang, X. F. J. Alloys Compd. 2011, 509, 740.
[37] Ahn, H.; Park, J. H.; Kim, S. B.; Jee, S. H.; Yoon, Y. S.; Kim, D. J. Electrochem. Solid-State Lett. 2010, 13, J125.
[38] Zhang, Z. Y.; Li, X. H.; Wang, C. H.; Wei, L. M.; Liu, Y. C.; Shao, C. L. J. Phys. Chem. C 2009, 113, 19397.
[39] Zhang, J.; Wang, S. R.; Xu, M. J.; Wang, Y.; Zhu, B. L.; Zhang, S. M.; Huang, W. P.; Wu, S. H. Cryst. Growth Des. 2009, 9, 3532.
[40] Wan, Q.; Li, Q. H.; Chen, Y. J.; Wang, Y. H.; He, X. L.; Li, J. P.; Lin, C. L. Appl. Phys. Lett. 2004, 84, 3654.
/
| 〈 |
|
〉 |