

A Novel Approach to Detect 2,4,6-trinitrotoluene/2,4,6-trinitrophenol Based on Fluorescence Quenching via Charge Transfer of Silicon Quantum Dots
Received date: 2014-03-20
Online published: 2014-04-25
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21025521, 21221003) and the Fundamental Research Funds for the Central University (No. 531107040687).
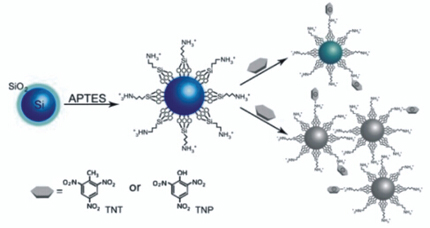
Due to the significant detrimental effects of nitroaromatic explosive on the environment and human health, sensitive, rapid, on-site and simple detection methods for explosives are in high demand. A novel label-free silicon quantum dots (SiQDs)-based sensor is designed for ultrasensitive detection of 2,4,6-trinitrotoluene (TNT) and 2,4,6-trinitrophenol (TNP). In this work, amino-functionalized SiQDs are obtained through treating the SiQDs with (3-aminopropyl)triethoxysilane (APTES). The amine-coated SiQDs are not only water-stable but also highly luminescent. Based on the dramatic and selective fluorescence quenching of the amine-coated SiQDs due to charge transfer that resulting from the formation of stable Meisenheimer complexes between electron-deficient TNT/TNP and electron-rich primary amine on the surface of SiQDs, an instant and sensitive sensor is developed for the detection of TNT/TNP which is able to not only directly suppress the fluorescent emission intensity of SiQDs but also induce SiQDs aggregation to result in self-quenching of SiQDs. The results reveal that the developed sensor has high sensitivity for the detection of TNT/TNP. As indicated in experimental results, the fluorescence intensity is proportional to the concentration of TNT/TNP. Meanwhile, linear correlations are obtained respectively for the fluorescence signals to the logarithmic TNT and TNP concentration with 6 and 7 order of range and the detection limit of TNT and TNP is 50 pg/mL for TNT and 5 pg/mL for TNP. Further experiments demonstrate that this analytical method is not susceptible to pH and prevent interference from other nitroaromatics or cationic ions. This simple and cost-effective sensor can be used to detect ultra-trace TNT/TNP in groundwater or seawater. Additionally, this sensor strategy may provide a convenient, rapid, highly sensitive and selective assay platform for nitroaromatic explosive.
Li Xiping , Liu Sijia , Wu Zhan , Jiang Jianhui . A Novel Approach to Detect 2,4,6-trinitrotoluene/2,4,6-trinitrophenol Based on Fluorescence Quenching via Charge Transfer of Silicon Quantum Dots[J]. Acta Chimica Sinica, 2014 , 72(5) : 563 -568 . DOI: 10.6023/A14030201
[1] Shriver-Lake, L. C.; Donner, B. L.; Ligler, F. S. Environ. Sci. Technol. 1997, 31(3), 837.
[2] Kim, T. H.; Lee, B. Y.; Jaworski, J.; Yokoyama, K.; Chung, W. -J.; Wang, E.; Hong, S.; Majumdar, A.; Lee, S.-W. ACS Nano 2011, 5(4), 2824.
[3] Toxicological Profile for 2,4,6-Trinitrotoluene, U. S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, 1995.
[4] Charles, P. T.; Adams, A. A.; Howell, P. B. Jr.; Trammell, S. A.; Deschamps, J. R.; Kusterbeck, A. W. Sensors 2010, 10(1), 876.
[5] Du, H. Y.; Ding, L. P.; Fang, Y. Chem. Online 2011, 74, 881. (杜海英, 丁立平, 房喻, 化学通报, 2011, 74, 881.)
[6] Rahimi-Nasrabadi, M.; Zahedi, M. M.; Pourmortazavi, S. M.; Heydari, R.; Rai, H.; Jazayeri, J.; Javidan, A. Microchim. Acta 2012, 177(1~2), 145.
[7] Berg, M.; Bolotin, J.; Hofstetter, T. B. Anal. Chem. 2007, 79(6), 2386.
[8] Dasary, S. S. R.; Singh, A. K.; Senapati, D.; Yu, H. T.; Ray, P. C. J. Am. Chem. Soc. 2009, 131(38), 13806.
[9] Zhang, C. L.; Li, Z.; Wu, Z. L.; Han, D. J. Spectroscopy Spect. Anal. 2012, 32(3), 686. (张春玲, 李喆, 吴正龙, 韩德俊, 光谱学与光谱分析, 2012, 32, 686.)
[10] Guerra-Diaz, P.; Gura, S.; Almirall, J. R. Anal. Chem. 2010, 82(7), 2826.
[11] Jiang, Y.; Zhao, H.; Zhu, N. N.; Lin, Y. Q.; Yu, P.; Mao, L. Q. Angew. Chem. 2008, 120(45), 8729.
[12] Ma, Y. X.; Li, H.; Peng, S.; Wang, L. Y. Anal. Chem. 2012, 84(19), 8415.
[13] Kartha, K. K.; Babu, S. S.; Srinivasan, S.; Ajayaghosh, A. J. Am. Chem. Soc. 2012, 134(10), 4834.
[14] Zhang, K.; Zhou, H. B.; Mei, Q. S.; Guan, G. J.; Liu, R. Y.; Zhang, J.; Zhang, Z. P. J. Am. Chem. Soc. 2011, 133(22), 8424.
[15] Goldman, E. R.; Medintz, I. L.; Whitley, J. L.; Hayhurst, A.; Clapp, A. R.; Uyeda, H. T.; Deschamps, J. R.; Lassman, M. E.; Mattoussi, H. J. Am. Chem. Soc. 2005, 127(18), 6744.
[16] Erogbogbo, F.; Yong, K.-T.; Roy, I.; Hu, R.; Law, W.-C.; Zhao, W. W.; Ding, H.; Wu, F.; Kumar, R.; Swihart, M. T.; Prasad, P. N. ACS Nano 2011, 5(1), 413.
[17] He, Y.; Fan, C. H.; Lee, S.-T. Nano Today 2010, 5(4), 282.
[18] Jurbergs, D.; Rogojina, E.; Mangolini, L.; Kortshagen, U. Appl. Phys. Lett. 2006, 88, 233116.
[19] Warner, J. H.; Hoshino, A.; Yamamoto, K.; Tilley, R. D. Angew. Chem. Int. Ed. 2005, 44(29), 4550.
[20] He, Y.; Kang, Z. H.; Li, Q. S.; Tsang, C. H. A.; Fan, C. H.; Lee, S. T. Angew. Chem., Int. Ed. 2009, 48(1), 128.
[21] Xie, C. G.; Liu, B. H.; Wang, Z. Y.; Gao, D. M.; Guan, G. J.; Zhang, Z. P. Anal. Chem. 2008, 80(2), 437.
[22] Gao, D. M.; Zhang, Z. P.; Wu, M. H.; Xie, C. G.; Guan, G. J.; Wang, D. P. J. Am. Chem. Soc. 2007, 129(25), 7859.
[23] Gao, D. M.; Wang, Z. Y.; Liu, B. H.; Ni, L.; Wu, M. H.; Zhang, Z. P. Anal. Chem. 2008, 80(22), 8545.
[24] Xu, S. F.; Lu, H. Z.; Li, J. H.; Song, X. L.; Wang, A. X.; Chen, L. X.; Han, S. B. ACS Appl. Mater. Interfaces 2013, 5(16), 8146.
[25] Kang, Z. H.; Liu, Y.; Tsang, C. H. A.; Ma, D. D. D.; Fan, X.; Wong, N. B.; Lee, S. T. Adv. Mater. 2009, 21(6), 661.
[26] Aptekar, J. W.; Cassidy, M. C.; Johnson, A. C.; Barton, R. A.; Lee, M. Y.; Ogier, A. C.; Vo, C.; Anahtar, M. N.; Ren, Y.; Bhatia, S. N.; Ramanathan, C.; Cory, D. G.; Hill, A. L.; Mair, R. W.; Rosen, M. S.; Walsworth, R. L.; Marcus1, C. M. ACS Nano 2009, 3(12), 4003.
[27] Cullis, A. G.; Canham, L. T. Nature 1991, 353, 335.
Sohn, H.; Calhoun, R. M.; Sailor, M. J.; Trogler, W. C. Angew. Chem., Int. Ed. 2001, 40(11), 2104.
/
| 〈 |
|
〉 |