

Recent Progress of Metal/Metal Oxide Nanoparticles for Asymmetric Hydrogenation and Transfer Hydrogenation
Received date: 2014-04-25
Online published: 2014-05-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21372118, 21232008), the National Basic Research Program of China(No. 2010CB833300), “333 High Level Talent Project”, “QingLan Project” of JiangSu Province and the Fundamental Research Funds for the Central Universities (NJAU) (No. KYRC201211).
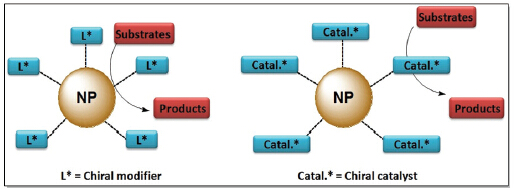
Metal/metal oxide nanoparticles for asymmetric hydrogenation and transfer hydrogenation have emerged as a frontier and evolved into a hot topic of asymmetric catalysis in recent years. Their catalytic modes resemble that of “nano-reactor”, where substrates diffuse through organic shells into catalytic active sites. Thus, high local catalyst concentration usually dramatically improves TON and TOF. In the case of nanoparticles as active sites, Orito’s platinum catalytic system received most extensive interests. Achievements have been made in chiral modifier structural modification, catalyst supports, reaction medium, nanoparticle morphology and catalytic mechanism. Moreover, other metal nanoparticles including palladium, rhodium, ruthenium, iridium and iron exhibited favorable catalytic efficiency in the asymmetric hydrogenation and transfer hydrogenation of alkenes, ketones and imines, especially for iridium and iron nanoparticles, ee values over 95% were obtained. In another case of metal/metal oxide nanoparticles as catalyst supports, comparable efficiency and enantioselectivity were observed to homogeneous catalysts, meanwhile, this protocol overcame the drawbacks of homogeneous catalysts with easier recovery and reuse. This review presents a brief overview on the recent progress in the asymmetric hydrogenation and transfer hydrogenation catalyzed by metal/metal oxide nanoparticles, as well as the related catalytic mechanism. However, there are still many challenges in this promising research field of metal/metal oxide nanoparticles for asymmetric catalysis. In addition to the continuous understanding of the catalytic mechanism, it is highly desirable to develop new types of metal/metal oxide nanoparticles with high efficiency, high enantioselectivity, and convenient recyclability.
Ji Yigang , Wu Lei , Fan Qinghua . Recent Progress of Metal/Metal Oxide Nanoparticles for Asymmetric Hydrogenation and Transfer Hydrogenation[J]. Acta Chimica Sinica, 2014 , 72(7) : 798 -808 . DOI: 10.6023/A14040325
[1] (a) Noyori, R. Asymmetric Catalysis in Organic Synthesis, Wiley, New York, 1994.
(b) Xie, J. H.; Zhou, Q. L. Acta Chim. Sinica 2012, 70, 1427. (谢建华, 周其林, 化学学报, 2012, 70, 1427.)
(c) Wang, D.; Hou, C. J.; Chen, L.-F.; Liu, X. N.; An, Q. D.; Hu, X. P. Chin. J. Org. Chem. 2013, 33, 1355. (王东, 候传金, 陈丽凤, 刘小宁, 安庆大, 胡向平, 有机化学, 2013, 33, 1355.)
(d) Cui, P. L.; Liu, H. M.; Guo, X. M.; Zhang, D. N.; Wang, Y. E.; Wang, C. Chin. J. Org. Chem. 2013, 33, 436. (崔朋雷, 刘卉敏, 果秀敏, 张冬暖, 王彦恩, 王春, 有机化学, 2013, 33, 436.)
(e) Li, X. N.; Zhang, P. L.; Duan, K.; Wang, J. X. Chin. J. Org. Chem. 2012, 32, 19. (李小娜, 张鹏亮, 段凯, 王家喜, 有机化学, 2012, 32, 19.)
(f) Liu, Y.; Wang, Z.; Ding, K. L. Acta Chim. Sinica 2012, 70, 1464. (刘龑, 王正, 丁奎岭, 化学学报, 2012, 70, 1464.)
[2] Knowles, W. S. Acc. Chem. Res. 1983, 16, 106.
[3] (a) Fan, Q. H.; Li, Y. M.; Chan, A. S. C. Chem. Rev. 2002, 102, 3385.
(b) Handbook of Asymmetric Heterogeneous Catalysis,Eds.: Ding, K.; Uozumi, Y., Wiley-VCH, 2008.
[4] (a) Wang, Z.; Chen, G.; Ding, K. L. Chem. Rev. 2009, 109, 322.
(b) Fan, Q. H.; Ding, K. L. Top. Organomet. Chem. 2011, 36, 207.
(c) Ma, B. D.; Deng, G. J.; Liu, J. ; He, Y. M.; Fan, Q. H. Acta Chim. Sinica 2013, 71, 528. (马保德, 邓国军, 刘继, 何艳梅, 范青华, 化学学报, 2013, 71, 528.)
(d) Liu, H. Q.; Shi, L.; Chen, Q. A.; Wang, L.; Zhou, Y. G. Acta Chim. Sinica 2013, 71, 40. (刘洪强, 时磊, 陈庆安, 王磊, 周永贵, 化学学报, 2013, 71, 40.)
[5] (a) Mallat, T.; Orglmeister, E.; Baiker, A. Chem. Rev. 2007, 107, 4863.
(b) Yasukawa, T.; Miyamura, H.; Kobayashi, S. Chem. Soc. Rev. 2014, 43, 1450.
[6] (a) Astruc, D.; Lu, F.; Aranzaes, J. R. Angew. Chem. Int. Ed. 2005, 44, 7852.
(b) Schätz, A.; Reiser, O.; Stark, W. J. Chem. Eur. J. 2010, 16, 8950.
[7] (a) Barbaro, P.; Santo, V. D.; Liguori, F. Dalton Trans. 2010, 39, 8391.
(b) Ranganath, K. V. S.; Glorius, F. Catal. Sci. Technol. 2011, 1, 13.
[8] Orito, Y.; Imai, S.; Niwa, S. Bull. Chem. Soc. Jpn. 1979, 1118.
[9] Harada, T.; Izumi, Y. Chem. Lett. 1978, 1195.
[10] Studer, M.; Blaser, H. U.; Exner, C. Adv. Synth. Catal. 2003, 345, 45.
[11] (a) Burgi, T.; Baiker, A. Acc. Chem. Res. 2004, 37, 909.
(b) Chen, C. H.; Zhan, E. S.; Li, Y.; Shen, W. J. J. Mol. Catal. A-Chem. 2013, 379, 117.
[12] (a) Griffiths, S. P.; Johnson, P.; Wells, P. B. Appl. Catal. A 2000, 191, 193.
(b) Chen, Z. J.; Li, X. H.; Li, C. Chin. J. Catal. 2011, 32, 155. (陈志坚, 李晓红, 李灿, 催化学报, 2011, 32, 155.)
[13] Li, X.; Shen, Y.; Xing, R.; Liu, Y. M.; Wu, H. H.; He, M. Y.; Wu, P. Catal. Lett. 2008, 122, 325.
[14] Xing, L.; Du, F.; Liang, J. J.; Chen, Y. S.; Zhou, Q. L. J. Mol. Catal. A-Chem. 2007, 276, 191.
[15] Li, B.; Li, X. H.; Wang, H. N.; Wu, P. J. Mol. Catal. A-Chem. 2011, 345, 81.
[16] Schmidt, E.; Vargas, A.; Mallat, T.; Baiker, A. J. Am. Chem. Soc. 2009, 131, 12358.
[17] Nandanan, E.; Gu, H. W.; Shao, H. L.; Jiang, J.; Ying, J. Y. Green Chem. 2011, 13, 3070.
[18] Beier, M. J.; Andanson, J.-M.; Mallat, T.; Krumeich, F.; Baiker, A. ACS Catal. 2012, 2, 337.
[19] Chen, Z. J.; Guan, Z. H.; Li, M. R.; Yang, Q. H.; Li, C. Angew. Chem. Int. Ed. 2011, 50, 4913.
[20] Guan, Z.; Lu, S.; Chen, Z.; Li, C.J. Catal. 2013, 305, 19.
[21] (a) Lavoie, S.; Mahieu, G.; McBreen, P. H. Angew. Chem. Int. Ed. 2006, 45, 7404.
(b) Lavoie, S.; Laliberté, M.-A.; Temprano, I.; McBreen, P. H. J. Am. Chem. Soc. 2006, 128, 7588.
[22] Schmidt, E.; Bucher, C.; Santarossa, G.; Mallat, T.; Gilmour, R.; Baiker, A. J. Catal. 2012, 289, 238.
[23] Meemken, F.; Maeda, N.; Hungerbühler, K.; Baiker, A. Angew. Chem. Int. Ed. 2012, 51, 8212.
[24] Perez, J. R. G.; Malthete, J.; Jacques, J. CR. Acad. Sc. Paris Serie Ⅱ . 1985, 300, 169.
[25] (a) Chen, C. H.; Zhan, E. S.; Li, Y.; Shen, W. J. Acta Chim. Sinica 2013, 71, 1505. (陈春辉, 展恩胜, 李勇, 申文杰, 化学学报, 2013, 71, 1505.)
(b) Chen, C. H.; Zhan, E. S.; Ta, N.; Li, Y.; Shen, W. J. Catal. Sci. Technol. 2013, 3, 2620.
[26] Huck, W.-R.; Mallat, T.; Baiker, A. New J. Chem. 2002, 26, 6.
[27] Nitta, Y.; Watanabe, J.; Okuyama, T.; Sugimura, T. J. Catal. 2005, 236, 164.
[28] Makra, Z.; Szöllösi, G.; Bartók, M. Catal. Today 2012, 181, 56.
[29] Maris, M.; Huck, W.-R.; Mallat, T.; Baiker, A. J. Catal. 2003, 219, 52.
[30] Watson, D. J.; Jesudason, R. J.; Beaumont, S. K.; Kyriakou, G.; Burton, J. W.; Lambert, R. M. J. Am. Chem. Soc. 2009, 131, 14584.
[31] Ma, H.; Chen, H.; Zhang, Q.; Li, X. J. Mol. Catal. A-Chem. 2003, 196, 131.
[32] Sonderegger, O. J.; Ho, G. M.-W.; Bürgi, T.; Baiker, A. J. Catal. 2005, 230, 499.
[33] Wang, J.; Feng, J.; Qin, R.; Fu, H.; Yuan, M.; Chen, H.; Li, X. Tetrahedron: Asymmetry 2007, 18, 1643.
[34] Jiang, H. Y.; Yang, C. F.; Li, C.; Fu, H. Y.; Chen, H.; Li, R. X.; Li, X. J. Angew. Chem. Int. Ed. 2008, 47, 1.
[35] Jiang, H. Y.; Sun, B.; Zheng, X. X.; Chen, H. Appl. Catal. A- Gen. 2012, 421, 86.
[36] (a) Johnson, N. B.; Lennon, I. C.; Moran, P. H.; Ramsden, J. A. Acc. Chem. Res. 2007, 40, 1291.
(b) Sun, Q.; Meng, X. J.; Liu, X.; Zhang, X. M.; Yang, Y.; Yang, Q. H.; Xiao, F. S. Chem. Commun. 2012, 48, 10505.
[37] Groves, J. T.; Myers, R. S. J. Am. Chem. Soc. 1983, 105, 5791.
[38] Gopalaiah, K. Chem. Rev. 2013, 113, 3248.
[39] (a) Mikhailine, A.; Lough, A. J.; Morris, R. H. J. Am. Chem. Soc. 2009, 131, 13943.
(b) Sonnenberg, J. F.; Coombs, N.; Dube, P. A.; Morris, R. H. J. Am. Chem. Soc. 2012, 134, 5893.
[40] Li, Y. Y.; Yu, S.; Wu, X.; Xiao, J.; Shen, W. Y.; Dong, Z. R.; Gao, J. X. J. Am. Chem. Soc. 2014, 136, 4031.
[41] Wu, L. Ph.D. Dissertation, Institute of Chemistry, Chinese Academy of Sciences (CAS), Beijing, 2007. (吴磊, 博士论文, 中国科学院化学研究所, 北京, 2007.)
[42] Li, H.; Luk, Y. Y.; Mrksich, M. Langmuir 1999, 15, 4957.
[43] Daniel, M. C.; Astruc, D. Chem. Rev. 2004, 104, 293.
[44] Belser, T.; Stöhr, M.; Pfaltz, A. J. Am. Chem. Soc. 2005, 127, 8720.
[45] Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H.; Bouhrara, M.; Basset, J. M. Chem. Rev. 2011, 111, 3036.
[46] Hu, A. G.; Yee, G. T.; Lin, W. B. J. Am. Chem. Soc. 2005, 127, 12486.
[47] Hu, A. G.; Liu, S.; Lin, W. B. RSC Adv. 2012, 2, 2576.
[48] Gao, X.; Liu, R.; Zhang, D.; Wu, M.; Cheng, T.; Liu, G. Chem. Eur. J. 2014, 20, 1515.
[49] Fraile, J. M.; García, J. I.; Mayoral, J. A. Chem. Rev. 2009, 109, 360.
[50] Wu, L.; He, Y. M.; Fan, Q. H. Adv. Synth. Catal. 2011, 353, 2915.
[51] Gleeson, O.; Davies, G. L.; Peschiulli, A.; Tekoriute, R.; Gun'ko, Y. K.; Connon, S. J. Org. Biomol. Chem. 2011, 9, 7929.
/
| 〈 |
|
〉 |