

Copper-Catalyzed Cyclization of Oxime Acetates with 2-Benzylidenemalononitriles for Synthesis of 2?Aminonicotinonitriles
Received date: 2014-04-09
Online published: 2014-06-05
Supported by
Project supported by Natural Science Foundation of China (No. 21172162), Young National Natural Science Foundation of China (No. 21202111) and Young Natural Science Foundation of Jiangsu Province (No. BK2012174).
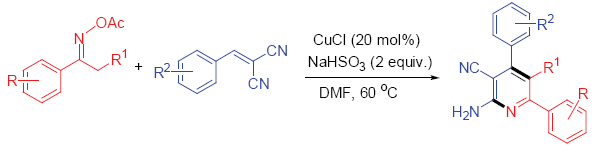
The 2-aminopyridine is the core structure of many potential drug candidates for the treatment of a variety of disorders. It is widely used as building block for the synthesis of series nitrogen-containing heterocycles. However, the traditional synthesis of 2-aminopyridines relies on amination of 2-unsubstituted pyridines with sodium amide which is limited to low yield and narrow scope for the using of strongly basic conditions. Oximes and their derivatives are a class of powerful building blocks in organic synthesis. Recently, a series of reports are focus on copper-catalyzed cyclization of oxime esters, and various methods are developed for the construction of five- or six-membered heterocycles. Based on our sustained efforts to copper-catalyzed cyclization for access to heterocyles, we herein report a novel process of copper-catalyzed cyclization of oxime esters with 2-benzylidenemalononitrile for the construction of 2-aminopyridines. Various 2-aminopyridines were obtained in middle to good yields under the optimized reaction conditions. In this case, the N—O bond cleavage would occur for oxime esters reduced by copper(I) catalyst, followed by the formation of nucleophilic copper(II) enamide which serve as a precursor in heterocycle synthesis. A representative procedure for the copper-catalyzed construction of 2-aminopyridines by cyclization of oxime esters with 2-benzylidenemalononitrile is as following: The substrate acetophenone O-acetyl oxime (1a, 0.5 mmol, 0.0885 g), 2-benzylidenemalononitrile (2a, 0.5 mmol, 0.0770 g), CuCl (0.1 mmol, 0.0989 g, 20 mol%), and NaHSO3 (1.0 mmol, 0.1041 g) were added to a 10 mL Schlenk tube, followed by addition of DMF (dried by calcium hydride and freshly distilled, 3 mL). The mixture was stirred at 60 ℃ as monitored by TLC. The solution was then quenched by H2O and extracted with EtOAc (20 mL×3), the combined organic layers were dried over Na2SO4, filtered, and evaporated under vaccum. The residue was purified by column chromatography on silica gel (eluent: light petroleum ether:ethyl acetate, V:V=5:1) to afford the desired product 4,6-diphenylpyridin-2-amine 3a.
Key words: copper; oxime esters; 2-benzylidenemalononitrile; cyclization; 2-aminopyridine
Cai Zhongjian , Lu Xinmou , Wang Shunyi , Ji Shunjun . Copper-Catalyzed Cyclization of Oxime Acetates with 2-Benzylidenemalononitriles for Synthesis of 2?Aminonicotinonitriles[J]. Acta Chimica Sinica, 2014 , 72(8) : 914 -919 . DOI: 10.6023/A14040263
[1] (a) Narasaka, K.; Kitamura, M. Eur. J. Org. Chem. 2005, 4505;
(b) Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V. V.; Noodleman, L.; Sharpless, K. B.; Fokin, V. V. J. Am. Chem. Soc. 2005, 127, 210;
(c) Alonso, D. A.; Nájera, C. Chem. Soc. Rev. 2010, 39, 2891;
(d) Sukhorukov, A. Y.; Ioffe, S. L. Chem. Rev. 2011, 111, 5004;
(e) Cai, H.; Li, D.; Liu, Z.; Wang, G. Acta Chim. Sinica 2013, 71, 717. (蔡海婷, 李丹丹, 刘姿, 王官武, 化学学报, 2013, 71, 717);
(f) Ran, L.; Liang, H.; Guan, Z. Chin. J. Org. Chem. 2013, 33, 66. (冉陇飞, 梁浩, 关正辉, 有机化学, 2013, 33, 66);
(g) Dai, H.; Yu, H.; Liu, J.; Qin, X.; Wang, T.; Zhang, X.; Qin, Z.; Fang, J. Chin. J. Org. Chem. 2013, 33, 1104. (戴红, 于海波, 刘建兵, 秦雪, 王婷婷, 张欣, 秦振芳, 方建新, 有机化学, 2013, 33, 1104);
(h) Liu, Z.; Wei, W.; Gan, C.; Huang, Y.; Liu, S.; Zhou, M.; Cui, J. Chin. J. Org. Chem. 2013, 33, 2551. (刘志平, 韦万兴, 甘春芳, 黄燕敏, 刘盛, 周敏, 崔建国, 有机化学, 2013, 33, 2551).
[2] (a) Ramalingan, C.; Park, Y.-T. J. Org. Chem. 2007, 72, 4536;
(b) Hashimoto, M.; Obora, Y.; Sakaguchi, S.; Ishii, Y. J. Org. Chem. 2008, 73, 2894;
(c) Ramón, R. S.; Bosson, J.; Díez-González, S.; Marion, N.; Nolan, S. P. J. Org. Chem. 2010, 75, 1197.
[3] (a) Choi, E.; Lee, C.; Na, Y.; Chang, S. Org. Lett. 2002, 4, 2369;
(b) De Luca, L.; Giacomelli, G.; Porcheddu, A. J. Org. Chem. 2002, 67, 6272;
(c) Augustine, J. K.; Atta, R. N.; Ramappa, B. K.; Boodappa, C. Synlett 2009, 3378;
(d) Zhu, J.-L.; Lee, F.-Y.; Wu, J.-D.; Kuo, C.-W.; Shia, K.-S. Synlett 2007, 1317.
[4] (a) Tanaka, K.; Kitamura, M.; Narasaka, K. Bull. Chem. Soc. Jpn. 2005, 78, 1659;
(b) Liu, S.; Yu, Y.; Libebeskind, L. S. Org. Lett. 2007, 9, 1947;
(c) Liu, S.; Liebeskind, L. S. J. Am. Chem. Soc. 2008, 130, 6918;
(e) Too, P. C.; Chua, S. H.; Wong, S. H.; Chiba, S. J. Org. Chem. 2011, 76, 6159.
[5] (a) Ren, Z.-H.; Zhang, Z.-Y.; Yang, B.-Q.; Wang, Y.-Y.; Guan, Z.-H. Org. Lett. 2011, 13, 5394;
(b) Ran, R.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. Green Chem. 2014, 16, 112.
[6] (a) Tang, X.; Huang, L.; Qi, C.; Wu, W.; Jiang, H. Chem. Commun. 2013, 49, 9597;
(b) Huang, H.; Ji, X.; Tang, X.; Zhang, M.; Li, X.; Jiang, H. Org. Lett. 2013, 15, 6254;
(c) Tang, X.; Huang, L.; Xu, Y.; Yang, J.; Wu, W.; Jiang, H. Angew. Chem., Int. Ed. DOI: 10.1002/anie.201311217.
[7] Wei, Y.; Yoshikai, N. J. Am. Chem. Soc. 2013, 135, 3756.
[8] (a) Deng, J.; Sanchez, T.; Al-Mawsawi, L. Q.; Dayam, R.; Yunes, R. A.; Garofalo, A.; Bolger, M. B.; Neamati, N. Bioorg. Med. Chem. 2007, 15, 4985;
(b) Fraley, A. W.; Chen, D.; Johnson, K.; McLaughlin, L. W. J. Am. Chem. Soc. 2003, 125, 616.
[9] Eri?, S.; Ke, S.; Barata, T.; Solmajer, T.; Stankovi?, J. A.; Jurani?, Z.; Savi?a, V.; Zloh, M. Bioorg. Med. Chem. 2012, 20, 5220.
[10] Laufer, S. A.; Zimmermann, W.; Ruff, K. J. J. Med. Chem. 2004, 47, 6311.
[11] Ji, H.; Delker, S. L.; Li, H.; Martásek, P.; Roman, L. J.; Poulos, T. L.; Silverman, R. B. J. Med. Chem. 2010, 53, 7804.
[12] Zong, R.; Zhou, H.; Thummel, R. P. J. Org. Chem. 2008, 73, 4334.
[13] (a) Chen, J.; Pang, Q.; Sun, Y.; Li, X. J. Org. Chem. 2011, 76, 3523;
(b) Chen, J.; Song, G.; Pan, C. L.; Li, X. Org. Lett. 2010, 12, 5426.
[14] Bentabed-Ababsa, G.; Ely, S. C. S.; Hesse, S.; Nassar, E.; Chevallier, F.; Nguyen, T. T.; Derdour, A.; Mongin, F. J. Org. Chem. 2010, 75, 839.
[15] (a) Hand, E. S.; Paudler, W. W. J. Org. Chem. 1978, 43, 2900;
(b) Denora, N.; Laquintana, V.; Pisu, M. G.; Dore, R.; Murru, L.; Latrofa, A.; Trapani, G.; Sanna, E. J. Med. Chem. 2008, 51, 6876;
(c) Yan, R.-L.; Yan, H.; Ma, C.; Ren, Z.-Y.; Gao, X.-A.; Huang, G.-S.; Liang, Y.-M. J. Org. Chem. 2012, 77, 2024;
(d) Zeng, J. Y.; Tan, J.; Leow, M. L.; Liu, X.-W. Org. Lett. 2012, 14, 4386;
(e) He, C.; Hao, J.; Xu, H.; Mo, Y.; Liu, H.; Han, J.; Lei, A. Chem. Commun. 2012, 48, 11073;
(f) Santra, S.; Bagdi, A. K.; Majee, A.; Hajra, A. Adv. Synth. Catal. 2013, 355, 1065;
(g) Bagdi, A. K.; Rahman, M.; Santra, S.; Majee, A.; Hajra, A. Adv. Synth. Catal. 2013, 355, 1741.
[16] Chichibabin, A. E.; Zeide, O. A. J. Russ. Phys. Chem. Soc. 1914, 46, 1216.
[17] (a) Cai, Z.-J.; Wang, S. Y.; Ji, S.-J. Org. Lett. 2012, 14, 6068;
(b) Cai, Z.-J.; Wang, S. Y.; Ji, S.-J. Adv. Synth. Catal. 2013, 355, 2686.
[18] Wu, Q.; Zhang, Y.; Cui, S. Org. Lett. 2014, 16, 1350.(a) Trost, B. M.; Rhee, Y. H. J. Am. Chem. Soc. 2002, 124, 2528;
(b) Nitta, M.; Iino, Y. Bull. Chem. Soc. Jpn. 1986, 59, 2365;
(c) Deeming, A. J.; Owen, D. W.; Powell, N. I. J. Organomet. Chem. 1990, 398, 299;
(d) Guan, Z.-H.; Zhang, Z.-Y.; Ren, Z.-H.; Wang, Y.-Y.; Zhang, X. J. Org. Chem. 2011, 76, 339.
/
| 〈 |
|
〉 |