

Supramolecular Self-Assembly of Cucurbit[8]uril with 2,2’-(Heptane-1,7-dily) Dibenzimidazolium Chloride
Received date: 2014-05-09
Online published: 2014-06-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272045), the Natural Science Foundation of the Department of Education of Guizhou Province (No. [2009]103) and the National Major Scientific Instruments Development Project (No. 2011YQ12003506).
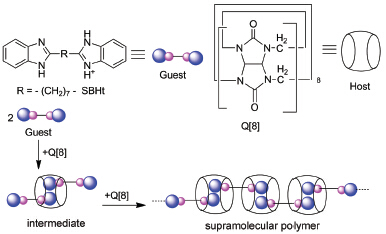
The interaction and corresponding supramolecular self-assembly of cucurbit[8]uril (Q[8]) with 2,2'-(heptane-1,7-dily)dibenzimidazolium chloride (SBHt) in solutions were investigated by means of 1H NMR spectroscopy, dynamic light scattering (DLS), ultraviolet absorption spectroscopy, fluorescence spectroscopy in details. The pKa shift of guest in the presence of Q[8] was first investigated in order to decide the pH of medium condition for investigation of interaction and corresponding supramolecular self-assembly of related host and guest. 1H NMR analysis revealed that the proton resonances of the aromatic ring move upfield of the unbound SBHt proton resonances, while the proton resonances of the alkyl chain move downfield of the unbound SBHt proton resonances, suggesting that interaction of Q[8] with SBHt could result in the formation of supramolecular polymer, and this suggestion was further confirmed by dynamic light scattering (DLS), ultraviolet absorption spectroscopy, fluorescence spectroscopy technologies. The average radius of the aggregates in the aqueous solution of Q[8]-SBHt is revealed to be over 100 nm. This gives an unambiguous evidence for the formation of a supramolecular architecture among Q[8] and SBHt. Moreover, the observed stability constants of Q[8]/SBHt host-guest complex were obtained by ultraviolet absorption spectroscopy, fluorescence spectroscopy technologies, they were 2.79×105 L/mol and 2.48×105 L/mol respectively. While the crystals structure analysis showed a simple 1:2 host-guest complex, which was different from that formed in solution. The competition of the outer-surface interaction of Q[n]s and the host-guest inclusion interaction could be the reason resulting in the formation of different self-assemblies of Q[8]/SBHt in solution and in solid state. In this particular case, the outer-surface interactions of Q[n]s include: 1) the C—H…π interaction between the waist methine groups or the bridged methylene groups on the outer surface of Q[8] molecule and the aromatic moiety in SBHt; 2) π…π stacking between portal carbonyl and the aromatic moiety in SBHt; moreover, 3) the unusual hydrogen bonding between the portal carbonyl oxygen of a Q[8] molecule and the waist methine groups or the bridged methylene groups from neighboring Q[8] molecule.
Yi Junming , Xiao Xin , Zhang Yunqian , Xue Saifeng , Tao Zhu , Zhang Jianxin . Supramolecular Self-Assembly of Cucurbit[8]uril with 2,2’-(Heptane-1,7-dily) Dibenzimidazolium Chloride[J]. Acta Chimica Sinica, 2014 , 72(8) : 949 -955 . DOI: 10.6023/A14050366
[1] Freeman, W. A.; Mock, W. L.; Shih, N.-Y. J. Am. Chem. Soc. 1981, 103, 7367.
[2] Day, A. I.; Arnold, A. P. WO 0068232, 2000,[Chem. Abstr. 2000, 133, P362775k].
[3] Kim, J.; Jung, I. S.; Kim, S. Y.; Lee, E.; Kang, J. K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. J. Am. Chem. Soc. 2000, 122, 540.
[4] Day, A. I.; Blanch, R. J.; Arnold, A. P.; Lorenzo, S.; Lewis, G. R.; Dance, I. Angew. Chem., Int. Ed. 2002, 41, 275.
[5] Cheng, X. J.; Liang, L. L.; Chen, K.; Ji, N. N.; Xiao, X.; Zhang, J. X.; Zhang, Y. Q.; Xue, S. F.; Zhu, Q. J.; Ni, X. L.; Tao, Z. Angew. Chem., Int. Ed. 2013, 52, 7252.
[6] Huang, Y.; Tao, Z.; Xue, S. F.; Zhu, Q. J. Chem. J. Chin. Univ. 2011, 32, 2022. (黄英, 陶朱, 薛赛凤, 祝黔江, 高等学校化学学报, 2011, 32, 2022.)
[7] Kim, K. Chem. Soc. Rev. 2002, 31, 96.
[8] Lee, J. W.; Samal, S.; Selvapalam, N.; Kim, H.-J.; Kim, K. Acc. Chem. Res. 2003, 36, 621.
[9] Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. Angew. Chem., Int. Ed. 2005, 44, 4844.
[10] Kim, K.; Selvapalam, N.; Ko, Y. H.; Park, K. M.; Kim, D.; Kim, J. Chem. Soc. Rev. 2007, 36, 267.
[11] Hwang, I.; Ziganshina, A. Y.; Ko, Y. H.; Yun, G.; Kim, K. Chem. Commun. 2009, 416.
[12] Xiao, X.; Wu, M. Q.; Xue, S. F.; Zhu, Q. J.; Zhang, J. X.; Tao, Z. Chin. J. Org. Chem. 2012, 33, 68. (肖昕, 吴明强, 薛赛凤, 祝黔江, 张建新, 陶朱, 有机化学, 2012, 33, 68.)
[13] Dong, N.; Wang, X. L.; Pan, J. P.; Tao, Z. Acta Chim. Sinica 2011, 69, 1431. (董南, 王秀林, 盘金品, 陶朱, 化学学报, 2011, 69, 1431.)
[14] Wang, Q. S.; Cong, H.; Zhang, J. X.; Tao, Z.; Xue, S. F. Chin. J. Org. Chem. 2011, 31, 1049. (王杞生, 丛航, 张建新, 陶朱, 薛赛凤, 有机化学, 2011, 31, 1049.)
[15] Dong, N.; Xue, S. F.; Tao, Z.; Zhao, Y.; Cai, J.; Liu, H. C. Acta Chim. Sinica 2008, 66, 117. (董南, 薛赛凤, 陶朱, 赵昱, 蔡洁, 刘洪材, 化学学报, 2008, 66, 117.)
[16] Zhu, G. M.; Yang, L. Y.; Cui, D. M. Chin. J. Org. Chem. 2014, 34, 495. (朱观明, 杨柳阳, 崔冬梅, 有机化学, 2014, 34, 495.)
[17] Peng, P.; Xiong, J. X.; Li, B.; Mo, G. Z.; Chen, R. H.; Wang, C. Y. Chin. J. Org. Chem. 2013, 33, 1891. (彭湃, 熊金锋, 李豹, 莫广珍, 陈任宏, 汪朝阳, 有机化学, 2013, 33, 1891)
[18] Mao, Z. Z.; Wang, C. Y.; Hou, M. N.; Song, X. M.; Luo, Y. F. Chin. J. Org. Chem. 2008, 28, 542. (毛郑州, 汪朝阳, 侯美娜, 宋秀美, 罗玉芬, 有机化学, 2008, 28, 542.)
[19] Biedermann, F.; Rauwald, U.; Cziferszky, M.; Williams, K. A.; Gann, L. D.; Guo, B. Y.; Urbach, A. R.; Bielawski, C. W.; Scherman, O. A. Chem. Eur. J. 2010, 16, 13716.
[20] Pischel, U.; Uzunova, V. D.; Remon, P.; Nau. W. M. Chem. Commun. 2010, 46, 2635.
[21] Yi, J. M.; Zhang, Y. Q.; Xue, S. F.; Zhu, Q. J. Acta Cryst. 2008, E64, o696.
[22] Sinha, M. K.; Reany, O.; Parvari, G.; Karmakar, A.; Keinan, E. Chem. Eur. J. 2010, 16, 9056.
[23] Zhang, Z. J.; Zhang, Y. M.; Liu, Y. J. Org. Chem. 2011, 76, 4682.
[24] Ge, J. Y.; Xue, S. F.; Zhu, Q. J.; Tao, Z.; Zhang, J. X. J. Inclusion Phenom. Macrocyclic Chem. 2007, 58, 63.
[25] De Greef, T. F.; Smulders, M. M. J.; Wolffs, M.; Schenning, A. P. H. J.; Sijbesma, R. P.; Meijer, E. W. Chem. Rev. 2009, 109, 5687.
[26] Aida, T.; Meijer, E. W.; Stupp, S. I. Science 2012, 335, 813.
[27] Harada, A.; Takashima, Y.; Yamaguchi, H. Chem. Soc. Rev. 2009, 38, 875.
[28] Dong, S.; Luo,Y.; Yan, X.; Zheng, B.; Ding, X.; Yu, Y.; Ma, Z.; Zhao, Q.; Huang, F. Angew. Chem., Int. Ed. 2011, 50, 1905.
[29] Zhang, Z.; Luo, Y.; Chen, J.; Dong, S.; Yu, Y.; Ma, Z.; Huang, F. Angew. Chem., Int. Ed. 2011, 50, 1397.
[30] Yan, X.; Xu, D.; Chi, X.; Chen, J.; Dong, S.; Ding, X.; Yu, Y.; Huang, F. Adv. Mater. 2012, 24, 362.
[31] Wang, F.; Han, C.; He, C.; Zhou, Q.; Zhang, J.; Wang, C.; Li, N.; Huang, F. J. Am. Chem. Soc. 2008, 130, 11254.
[32] Wang, F.; Zhang, J.; Ding, X.; Dong, S.; Liu, M.; Zheng, B.; Li, S.; Wu, L.; Yu, Y.; Gibson, H. W.; Huang, F. Angew. Chem., Int. Ed. 2010, 49, 1090.
[33] Zhang, M.; Xue, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Angew. Chem., Int. Ed. 2012, 51, 7011.
[34] Ji, X.; Yao, Y.; Li, J.; Yan, X.; Huang, F. J. Am. Chem. Soc. 2013, 135, 74.
[35] Ji, X.; Dong, S.; Wei, P.; Xia, D.; Huang, F. Adv. Mater. 2013, 25, 5725.
[36] Zhang, J.; Coulston, R. J.; Jones, S. T.; Geng, J.; Scherman, O. A.; Abell, C. Science 2012, 335, 690.
[37] Liu, Y. L.; Huang, Z. H.; Tan, X. X.; Wang, Z. Q.; Zhang, X. Chem. Commun. 2013, 49, 5766.
[38] Tan, X. X.; Yang, L.L.; Liu, Y. L.; Huang, Z. H.; Yang, H.; Wang, Z. Q.; Zhang, X. Polym. Chem. 2013, 4, 5378.
[39] Liu, Y. L.; Huang, Z. H.; Liu, K.; Kelgtermans, H.; Dehaen, W.; Wang, Z. Q.; Zhang, X. Polym. Chem. 2014, 5, 53.
[40] Yang, L. L.; Liu, X. G.; Tan, X. X.; Yang, H.; Wang, Z. Q.; Zhang, X. Polym. Chem. 2014, 5, 323.
[41] Yang, H.; Ma, Z.; Wang, Z. Q.; Zhang, X. Polym. Chem. 2014, 5, 1471.
[42] Li, L. F.; Lin, Y. W.; Huang, Z. W.; Luo, H. B. Chem. J. Chin. Univ. 2012, 33, 282. (李立凡, 林友文, 黄智文, 罗红斌, 高等学校化学学报, 2012, 33, 282.)Ni, X. L.; Xiao, X.; Cong, H.; Zhu, Q. J.; Xue, S. F.; Tao, Z. Acc. Chem. Res. 2014, 47, 1386.
/
| 〈 |
|
〉 |