

Chiral Phosphite-Olefin Ligands:Application in Rh-Catalyzed Asymmetric 1,4-Addition of Arylboronic Acids to β-Aryl-α,β-unsaturated Sulfonates
Received date: 2014-06-04
Online published: 2014-06-26
Supported by
Project supported by the National Natural Science Foundation of China (No. 21325209), the Shanghai Municipal Committee of Science and Technology (No. 14XD1404400).
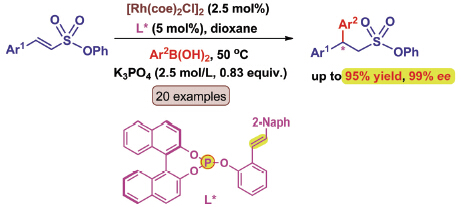
Chiral sulfonyl compounds have great versatility in organic synthesis, and they are also important as biologically active substances in medicinal chemistry. Among various methods developed for their synthesis, rhodium-catalyzed asymmetric 1,4-addition of arylboronic acids to α,β-unsaturated sulfonyl compounds represents one of the most practical methods due to the stability and availability of the boronic acid used as a nucleophile. Although several Rh(I) complexes of bidentate ligands have been discovered for asymmetric conjugation addition of α,β-unsaturated sulfonyl compounds, some challenging issues still remain in terms of efficiency, enantioselectivity and substrate scope. Therefore, the development of an efficient catalytic system for the synthesis of chiral sulfonyl compounds is an important goal in extending the current methodology. Here, a general and mild method for the rhodium-catalyzed enantioselective catalytic conjugate addition of arylboronic acids to β-aryl-α,β-unsaturated sulfonate is described. The success of the process relies on the use of extraordinary simple chiral phosphite-olefin ligands as bidentate ligands which offer notable synthetic and economic advantages. Optimum reaction condition was determined to run the reaction at 50 ℃ using dioxane as the solvent, in the presence of 2.5 mol% of [Rh(coe)2Cl]2 and 5 mol% of chiral P/olefin ligand L7. This Rh(I) catalyst containing chiral P/olefin ligand has a broad substrate scope, a wide range of arylboronic acids with varying electronic and steric demands were successfully examined with α,β-unsaturated sulfonate (1). Notably, all transformations proceed efficiently to give the desired products in good yields (84%~95%) and excellent selectivities (92%~99% ee). The electronic properties of the arylboronic acids did not appear to affect the reactivity of the reaction. Besides, α,β-unsaturated sulfonate 1 with either an electron-donating or electron-withdrawing group on any aromatic carbon readily underwent the asymmetric arylation with arylboronic acids, affording chiral sulfonates in high yields and enantioselectivities. The current reaction provides a practical approach to the synthesis of diverse highly enantioenriched gem-diaryl substituted sulfonates.
Yu Yue-Na , Xu Ming-Hua . Chiral Phosphite-Olefin Ligands:Application in Rh-Catalyzed Asymmetric 1,4-Addition of Arylboronic Acids to β-Aryl-α,β-unsaturated Sulfonates[J]. Acta Chimica Sinica, 2014 , 72(7) : 815 -819 . DOI: 10.6023/A14060436
[1] (a) Perlmutter, P. Conjugate Addition Reactions in Organic Synthesis;Tetrahedron Organic Chemistry Series 9, Pergamon Press, Oxford, U. K., 1992.
(b) Rossiter, B. E.; Swingle, N. M. Chem. Rev. 1992, 92, 771.
(c) Zhang, Z.; Xie, F.; Yang, B.; Yu, H.; Zhang, W. Chin. J. Org. Chem. 2011, 31, 429. (张振锋, 谢芳, 杨波, 余焓, 张万斌, 有机化学, 2011, 31, 429.)
(d) Ying, A.; Wu, C.; Fu, Y.; Ren, S.; Liang, H. Chin. J. Org. Chem. 2012, 32, 1587. (应安国, 武承林, 付永前, 任世斌, 梁华定, 有机化学, 2012, 32, 1587.)
[2] For reviews, see: (a) Tomioka, K.; Nagaoka, Y. Comprehensive Asymmetric Catalysis, Eds.: Jacobsen, E. N.; Pfaltz, A.; Yamamoto, H., Springer, New York, 1999, p. 1105
(b) Feringa, B. L. Acc. Chem. Res. 2000, 33, 346.
(c) Krause, N.; Hoffmann-Röder, A. Synthesis 2001, 2, 171.
(d) Feringa, B. L.; Naasz, R.; Imbos, R.; Arnold, L. A. In Modern Organocopper Chemistry, Ed.: Krause, N., VCH, Weinheim, Germany, 2002, p. 224.
(e) Alexakis, A.; Benhaim, C. Eur. J. Org. Chem. 2002, 67, 3221.
(f) Hayashi, T.; Yamasaki, K. Chem. Rev. 2003, 103, 2829.
(g) Woodward, S. Angew. Chem., Int. Ed. 2005, 44, 5560.
(h) López, F.; Minnaard, A. J.; Feringa, B. L. Acc. Chem. Res. 2007, 40, 179.
(i) López, F.; Minnaard, A. J.; Feringa, B. L. In The Chemistry of Organomagnesium Compounds, Eds.: Rappoport, Z.; Marek, I., Wiley, Chichester, U. K., 2008; Part 2, Chapter 17.
(j) Harutyunyan, S. R.; den Hartog, T.; Geurts, K.; Minaard, A. J.; Feringa, B. L. Chem. Rev. 2008, 108, 2824.
[3] For reviews, see: (a) Tian, P.; Dong, H.-Q.; Lin, G.-Q. ACS Catal. 2012, 2, 95.
(b) Partyka, D. V. Chem. Rev. 2011, 111, 1529.
(c) Berthon, G.; Hayashi, T. In Catalytic Asymmetric Conjugate Reactions, Ed.: Córdova, A., Wiley-VCH, Weinheim, Germany, 2010, Chapter 1, p. 1.
(d) Edwards, H. J.; Hargrave, J. D.; Penrose, S. D.; Frost, C. G. Chem. Soc. Rev. 2010, 39, 2093.
(e) Johnson, J. B.; Rovis, T. Angew. Chem., Int. Ed. 2008, 47, 840.
(f) Darses, S.; Genet, J.-P. Eur. J. Org. Chem. 2003, 4313.
(g) Hayashi, T.; Yamasaki, K. Chem. Rev. 2003, 103, 2829.
(h) Fagnou, K.; Lautens, M. Chem. Rev. 2003, 103, 169.
(i) Bolm, C.; Hildebrand, J. P.; Muñiz, K.; Hermanns, N. Angew. Chem., Int. Ed. 2001, 40, 3284.
(j) Christoffers, J.; Koripelly, G.; Rosiak, A.; Rössle, M. Synthesis 2007, 1279.
(k) Enders, D.; Lüttgen, K.; Narine, A. A. Synthesis 2007, 959.
(l) Hayashi, T. Synlett 2001, 879.
(m) Hayashi, T.; Yamasaki, K. Chem. Rev. 2003, 103, 2829.
(n) Shintani, R.; Tokunaga, N.; Doi, H.; Hayashi, T. J. Am. Chem. Soc. 2004, 126, 6240.
[4] Takaya, Y.; Ogasawara, M.; Hayashi, T.; Sakai, M.; Miyaura, N. J. Am. Chem. Soc. 1998, 120, 5579.
[5] Early studies of α,β-unsaturated ketones, see: (a) Reetz, M. T.; Moulin, D.; Gosberb, A. Org. Lett. 2001, 3, 4083.
(b) Kuriyama, M.; Nagai, K.; Yamada, K.-i.; Miwa, Y.; Taga, T.; Tomioka, K. J. Am. Chem. Soc. 2002, 124, 8932.
(c) Boiteau, J. G.; Imbos, R.; Minnaard, A. J.; Feringa, B. L. Org. Lett. 2003, 5, 681.
(d) Iguchi, Y.; Itooka, R.; Miyaura, N. Synlett 2003, 1040.
(e) Defieber, C.; Paquin, J.-F.; Serna, S.; Carreira, E. M. Org. Lett. 2004, 6, 3873.
[6] α,β-Unsaturated Esters, Amides, and Aldehydes: (a) Takaya, Y.; Senda, T.; Kurushima, H.; Ogasawara, M.; Hayashi, T. Tetrahedron: Asymmetry 1999, 10, 4047.
(b) Sakuma, S.; Sakai, M.; Itooka, R.; Miyaura, N. J. Org. Chem. 2000, 65, 5951.
(c) Senda, T.; Ogasawara, M.; Hayashi, T. J. Org. Chem. 2001, 66, 6852.
(d) Sakuma, S.; Miyaura, N. J. Org. Chem. 2001, 66, 8944.
(e) Paquin, J.-F.; Defieber, C.; Stephenson, C. R. J.; Carreira, E. M. J. Am. Chem. Soc. 2005, 127, 10850.
[7] Alkenylphosphonates: Hayashi, T.; Senda, T.; Takaya, Y.; Ogasawara, M. J. Am. Chem. Soc. 1999, 121, 11591.
[8] Nitroalkenes: Hayashi, T.; Senda, T.; Ogasawara, M. J. Am. Chem. Soc. 2000, 122, 10716.
[9] Alkenylheteroarenes and alkenylarenes: (a) Pattison, G.; Piraux, G.; Lam, H. W. J. Am. Chem. Soc. 2010, 132, 14373.
(b) Saxena, A.; Lam, H. W. Chem. Sci. 2011, 2, 2326.
[10] For reviews, see: (a) Simpkins, N. S. Tetrahedron 1990, 46, 6951.
(b) Rayner, C. M. Contemp. Org. Synth. 1996, 3, 499.
(c) Nájera, C.; Yus, M. Tetrahedron 1999, 55, 10547.
(d) Nájera, C.; Yus, M. Tetrahedron 1999, 55, 10547.
(e) Bäckvall, J.-E.; Chinchilla, R.; Nájera, C.; Yus, M. Chem. Rev. 1998, 98, 2291.
(f) Meadows, D. C.; Gervay-Hague, J. Med. Res. Rev. 2006, 26, 793.
(j) Tozer, M. J.; Harper, E. A.; Kalindjian, S. B.; Pether, M. J.; Shankley, N. P.; Watt, G. F. Bioorg. Med. Chem. Lett. 1999, 9, 1825.
(h) Tamamura, H.; Koh, Y.; Ueda, S.; Sasaki, Y.; Yamasaki, T.; Aoki, M.; Maeda, K.; Watai, Y.; Arikuni, H.; Otaka, A.; Mitsuya, H.; Fujii, N. J. Med. Chem. 2003, 46, 1764.
(i) Hanessian, S.; Sailes, H.; Therrien, E. Tetrahedron 2003, 59, 7047.
(j) Zajac, M.; Peters, R. Chem. Eur. J. 2009, 15, 8204.
[11] For recent examples, see: (a) Enders, D.; Müller, S. F.; Raabe, G. Angew. Chem., Int. Ed. 1999, 38, 195.
(b) Grimaud, L.; Rotulo, D.; Ros-Perez, R.; Guitry-Azam, L.; Prunet, J. Tetrahedron Lett. 2002, 43, 7477.
(c) Luis, L. A.; Krische, M. J. Synthesis 2004, 2579.
(d) Tsui, G. C.; Lautens, M. Angew. Chem., Int. Ed. 2010, 49, 8938.
(e) García Ruano, J. L.; Schöpping, C.; Alvarado, C.; Alemán, J. Chem. Eur. J. 2010, 16, 8968.
(f) So, C. M.; Kume, S.; Hayashi, T. J. Am. Chem. Soc. 2013, 135, 10990.
(g) Lu, J.; Ye, J.; Duan, W. Chem. Commun. 2014, 50, 698.
[12] For examples of copper-catalyzed asymmetric transformations, see: (a) Llamas, T.; Arrayás, R. G.; Carretero, J. C. Angew. Chem., Int. Ed. 2007, 46, 3329.
(b) Desrosiers, J.-N.; Charette, A. B. Angew. Chem., Int. Ed. 2007, 46, 5955.
(c) Bechara, W. S.; Charette, A. B. Org. Lett. 2008, 10, 2315.
(d) Bos, P. H.; Minnaard, A. J.; Feringa, B. L. Org. Lett. 2008, 10, 4219.
(e) Bos, P. H.; Maciá, B.; Fernández-Ibáñez, M.Á.; Minnaard, A. J.; Feringa, B. L. Org. Biomol. Chem. 2010, 8, 47.
[13] For a review of organocatalytic asymmetric addition to alkenyl sulfones, see: Nielsen, M.; Jacobsen, C. B.; Holub, N.; Paixão, M. W.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2010, 49, 2668.
[14] (a) Mauleón, P.; Carretero, J. C. Org. Lett. 2004, 6, 3195.
(b) Mauleón, P.; Carretero, J. C. Chem. Commun. 2005, 41, 4961.
(c) Mauleón, P.; Alonso, I.; Rivero, M. R.; Carretero, J. C. J. Org. Chem. 2007, 72, 9924.
[15] Nishimura, T.; Takiguchi, Y.; Hayashi, T. J. Am. Chem. Soc. 2012, 134, 9086.
[16] (a) Jin, S.-S.; Wang, H.; Xu, M.-H. Chem. Commun. 2011, 47, 7230.
(b) Qi, W.-Y.; Zhu, T.-S.; Xu, M.-H. Org. Lett. 2011, 13, 3410.
(c) Jin, S.-S.; Wang, H.; Zhu, T.-S.; Xu, M.-H. Org. Biomol. Chem. 2012, 10, 1764.
(d) Zhu, T.-S.; Jin, S.-S.; Xu, M.-H. Angew. Chem., Int. Ed. 2012, 51, 780.
(e) Wang, H.; Zhu, T.-S.; Xu, M.-H. Org. Biomol. Chem. 2012, 10, 9158.
(f) Zhu, T.-S.; Chen, J.-P.; Xu, M.-H. Chem. Eur. J. 2013, 19, 865.
(g) Wang, H.; Jiang, T.; Xu, M.-H. J. Am. Chem. Soc. 2013, 135, 971.
(h) Wang, H.; Xu, M.-H. Synthesis 2013, 45, 2125.
(i) Li, Y.; Zhu, D.-X.; Xu, M.-H. Chem. Commun. 2013, 49, 11659.
(j) Li, Y.; Xu, M.-H. Chem. Commun. 2014, 50, 3771.
[17] Yu, Y.-N.; Xu, M.-H. Org. Chem. Front. 2014, DOI: 10. 1039/c4qo00135d.
[18] Selected early examples of the use of chiral phosphorus-based olefin ligands: (a) Maire, P.; Deblon, S.; Breher, F.; Geier, J.; Böhler, C.; Rügger, H.; Schönberg, H.; Grtrümacher, H. Chem. Eur. J. 2004, 10, 4198.
(b) Shintani, R.; Duan, W.-L.; Nagano, T.; Okada, A.; Grützmacher, T. Angew. Chem., Int. Ed. 2005, 44, 4611.
(c) Defieber, C.; Ariger, M. A.; Moriel, P.; Carreira, E. M. Angew. Chem. Int. Ed. 2007, 46, 3139.
(d) Mariz, R.; Briceño, A.; Dorta, R.; Dorta, R. Organometallics 2008, 27, 6605.
(e) Liu, Z.; Du, H. Org. Lett. 2010, 12, 3054.
/
| 〈 |
|
〉 |