

A New Approach for Supramolecular Iminium Catalysis
Received date: 2014-04-30
Online published: 2014-06-24
Supported by
Project supported by National Natural Science Foundation of China (No. 21372105), Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1138), the International S&T Cooperation Program of China (No. 2013DFR70580), the National Basic Research Program of China (No. 2010CB833203), and the “111” Program from MOE of P.R. China.
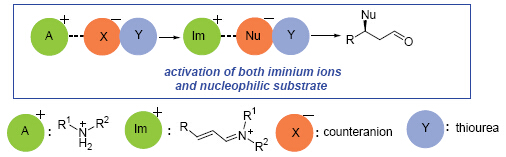
An alternative modular strategy has been developed for hydrogen-bond-mediated supramolecular iminium catalysis (SIC). To improve the efficiency of traditional iminium ion catalysis and provide a new approach to asymmetric catalysis, we recently developed a new concept which aims to activate iminium ions. To expand the scope of SIC, we reported here a rationally designed strategy involving the dual activation of both iminium ions and the nucleophilic partner that provides new opportunities for improving the reactivity and designing new reactions. We analyzed the different reaction rates of the addition reaction of malonate to α,β-unsaturated aldehyde catalyzed by traditional iminium ion catalysis and supramolecular iminium catalysis by the means of 1H NMR monitoring of the reaction conversions and comparison of the isolated yields. Moreover, different carboxylic acids were employed in iminium catalytic Michael addition reaction of malonate to α,β-unsaturated aldehyde to test our hypothesis. General speaking, by using this novel supramolecular iminium catalysis, the enantioselectivities of the Michael addition products are excellent (93%~95%) with moderate to good yields (61%~86%), meanwhile, all these reactions were rather slow and only 33%~57% conversions were obtained even after 7 days in the presence of traditional iminium ion catalysis. Further reducing the catalyst loading to 5 mol%, the reaction proceeded smoothly to give a >90% conversion with 94% ee after 12 h. The results revealed that the use of less acidic acid which generates lower concentration of iminium ion associated with stronger anion-binding stabilized conjugate base gives faster reaction rate. The experimental results indicate a new approach for SIC which involves an anion-exchange process between anion-binding stabilized carboxylate ion and malonate. The self-assembled supramolecular system has higher reactivity, better efficiency and greater turnover numbers. This general strategy has the potential to be applied in many iminium catalytic nucleophilic addition reactions and might provide new opportunities for designing new asymmetric reactions. The further study is currently underway in our laboratory.
Yu Tianyang , Wang Yao , Xu Pengfei . A New Approach for Supramolecular Iminium Catalysis[J]. Acta Chimica Sinica, 2014 , 72(7) : 845 -848 . DOI: 10.6023/A14040342
[1] (a) Wang, H.; He, Q.; Tan, K. Acta Chim. Sinica 2013, 71, 1663. (王红, 何桥, 谭凯, 化学学报, 2013, 71, 1663.);
(b) Chen, C.; Zhan, E.; Li, Y.; Shen, W. Acta Chim. Sinica 2013, 71, 1505. (陈春辉, 展恩胜, 李勇, 申文杰, 化学学报, 2013, 71, 1505.);
(c) Du, L.; Cao, P.; Liao, J. Acta Chim. Sinica 2013, 71, 1239. (杜乐, 曹鹏, 廖建, 化学学报, 2013, 71, 1239.);
(d) Ma, F.; Chen, J. Acta Chim. Sinica 2013, 71, 1118. (马菲, 陈建新, 化学学报, 2013, 71, 1118.);
(e) Wu, X.; Li, M.; Gong, L. Acta Chim. Sinica 2013, 71, 1091. (吴祥, 李明丽, 龚流柱, 化学学报, 2013, 71, 1091.);
(f) Hu, R.; Zhang, C.; Pei, Z. Acta Chim. Sinica 2013, 71, 1064. (胡锐, 张承平, 裴志胜, 化学学报, 2013, 71, 1064.);
(g) Huang, J.; Luo, S.; Gong, L. Acta Chim. Sinica 2013, 71, 879. (黄建洲, 罗时玮, 龚流柱, 化学学报, 2013, 71, 879.);
(h) He, Z.; Huang, Y.; Verpoort, F. Acta Chim. Sinica 2013, 71, 700. (何展荣, 黄毅勇, Verpoort Francis, 化学学报, 2013, 71, 700.);
(i) Ma, B.; Deng, G.; Liu, J.; He, Y.; Fan, Q. Acta Chim. Sinica 2013, 71, 528. (马保德, 邓国军, 刘继, 何艳梅, 范青华, 化学学报, 2013, 71, 528.);
(j) Qiu, H.; Zhang, D.; Liu, S.; Qiu, L.; Zhou, J.; Qian, Y.; Zhai, C.; Hu, W. Acta Chim. Sinica 2012, 70, 2484. (邱晃, 张丹, 刘顺英, 邱林, 周俊, 钱宇, 翟昌伟, 胡文浩, 化学学报, 2012, 70, 2484.);
(k) Zheng, K.; Lin, L.; Feng, X. Acta Chim. Sinica 2012, 70, 1785. (郑柯, 林丽丽, 冯小明, 化学学报, 2012, 70, 1785.);
(l) Sun, X.; Peng, J.; Zhang, S.; Zhou, Q.; Dong, L.; Chen, Y. Acta Chim. Sinica 2012, 70, 1682. (孙峋皓, 彭景, 张叔阳, 周清清, 董琳, 陈应春, 化学学报, 2012, 70, 1682.);
(m) Lv, J.; Zhong, X.; Cheng, J.-P.; Luo, S. Acta Chim. Sinica 2012, 70, 1518. (吕健, 钟兴仁, 程津培, 罗三中, 化学学报, 2012, 70, 1518.);
(n) Liu, Y.; Wang, Z.; Ding, K. Acta Chim. Sinica 2012, 70, 1464. (刘龑, 王正, 丁奎岭, 化学学报, 2012, 70, 1464.);
(o) Liu, Y.; Zhou, J. Acta Chim. Sinica 2012, 70, 1451. (刘运林, 周剑, 化学学报, 2012, 70, 1451.);
(p) Xie, J.; Zhou, Q. Acta Chim. Sinica 2012, 70, 1427. (谢建华, 周其林, 化学学报, 2012, 70, 1427.)
[2] Vogl, E. M.; Gröger, H.; Shibasaki, M. Angew. Chem., Int. Ed. 1999, 38, 1570.
[3] For selected reviews, see: (a) Lelais, G.; MacMillan, D. W. C. Aldrichim. Acta 2006, 39, 79;
(b) List, B. Chem. Commun. 2006, 819;
(c) Dalko, P. I. Enantioselective Organocatalysis, Wiley-VCH, Weinheim, 2007;
(d) Erkkilä, A.; Majander, I.; Pihko, P. M. Chem. Rev. 2007, 107, 5416;
(e) Dondoni, A.; Massi, A. Angew. Chem. Int. Ed. 2008, 47, 4638;
(f) MacMillan, D. W. C. Nature 2008, 455, 304;
(g) Melchiorre, P.; Marigo, M.; Carlone, A.; Bartoli, G. Angew. Chem. Int. Ed. 2008, 47, 6138;
(h) Bertelsen, S.; Jørgensen, K. A. Chem. Soc. Rev. 2009, 38, 2178;
(i) Marqués-López, E.; Herrerabc, R. P.; Christmann, M. Nat. Prod. Rep. 2010, 27, 1138;
(j) Grondal1, C.; Jeanty, M.; Enders, D. Nat. Chem. 2010, 2, 167;
(k) Nielsen, M.; Worgull, D.; Zweifel, T.; Gschwend, B.; Bertelsen, S.; Jørgensen, K. A. Chem. Commun. 2011, 47, 632.
[4] Wang, Y.; Yu, T.-Y.; Zhang, H.-B.; Luo, Y.-C.; Xu, P.-F. Angew. Chem., Int. Ed. 2012, 51, 12339.
[5] For selected reviews on anion-binding chemistry, see: (a) Caltagirone, C.; Gale, P. A. Chem. Soc. Rev. 2009, 38, 520;
(b) Kubik, S. Chem. Soc. Rev. 2009, 38, 585;
(c) Zhang, Z.; Schreiner, P. R. Chem. Soc. Rev. 2009, 38, 1187. For selected examples of thiourea anion-binding catalysis, see:
(d) Kotke, M.; Schreiner, P. R. Tetrahedron 2006, 62, 434;
(e) Kotke, M.; Schreiner, P. R. Synthesis 2007, 779;
(f) Raheem, I. T.; Thiara, P. S.; Peterson, E. A.; Jacobsen, E. N. J. Am. Chem. Soc. 2007, 129, 13404;
(g) Reisman, S. E.; Doyle, A. G.; Jacobsen, E. N. J. Am. Chem. Soc. 2008, 130, 7198;
(h) De, C. K.; Klauber, E. G.; Seidel, D. J. Am. Chem. Soc. 2009, 131, 17060;
(i) Zuend, S. J.; Coughlin, M. P.; Lalonde, M. P.; Jacobsen, E. N. Nature 2009, 461, 968;
(j) Xu, H.; Zuend, S. J.; Woll, M. G.; Tao, Y.; Jacobsen, E. N. Science 2010, 327, 986;
(k) Brown, A. R.; Kuo, W.-H.; Jacobsen, E. N. J. Am. Chem. Soc. 2010, 132, 9286;
(l) Singh, R. P.; Foxman, B. M.; Deng, L. J. Am. Chem. Soc. 2010, 132, 9558;
(m) Klauber, E. G.; De, C. K.; Shah, T. K.; Seidel, D. J. Am. Chem. Soc. 2010, 132, 13624;
(n) Birrell, J. A.; Desrosiers, J.-N.; Jacobsen, E. N. J. Am. Chem. Soc. 2011, 133, 13872;
(o) Burns, N. Z.; Witten, M. G.; Jacobsen, E. N. J. Am. Chem. Soc. 2011, 133, 14578;
(p) Zhu, R.-X.; Zhang, D.-J.; Wang, R.-X.; Liu, C.-B. Acta Chim. Sinica 2008, 66, 885. (朱荣秀, 张冬菊, 王若曦, 刘成卜, 化学学报, 2008, 66, 885.);
(q) Zhu, Y.; Zhang, X.; Zhang, Q.; Dai, X.; Wu, J. Acta Chim. Sinica 2011, 69, 709. (朱玉莲, 张小燕, 张岐, 戴雪芹, 吴静波, 化学学报, 2011, 69, 709.)
[6] (a) Brandau, S.; Landa, A.; Franzen, J.; Marigo, M.; Jørgensen, K. A. Angew. Chem. Int. Ed. 2006, 45, 4305;
(b) Wang, Y. C.; Li, P. F.; Liang, X. M.; Ye, J. X. Adv. Synth. Catal. 2008, 350, 1383.
[7] Bwdwell, F. G.; Algrim, D. J. Org. Chem. 1976, 41, 2507.
/
| 〈 |
|
〉 |